Figure 7. Possible structural consequences of L718V/Q-mutations for EGFR binding to TKIs.
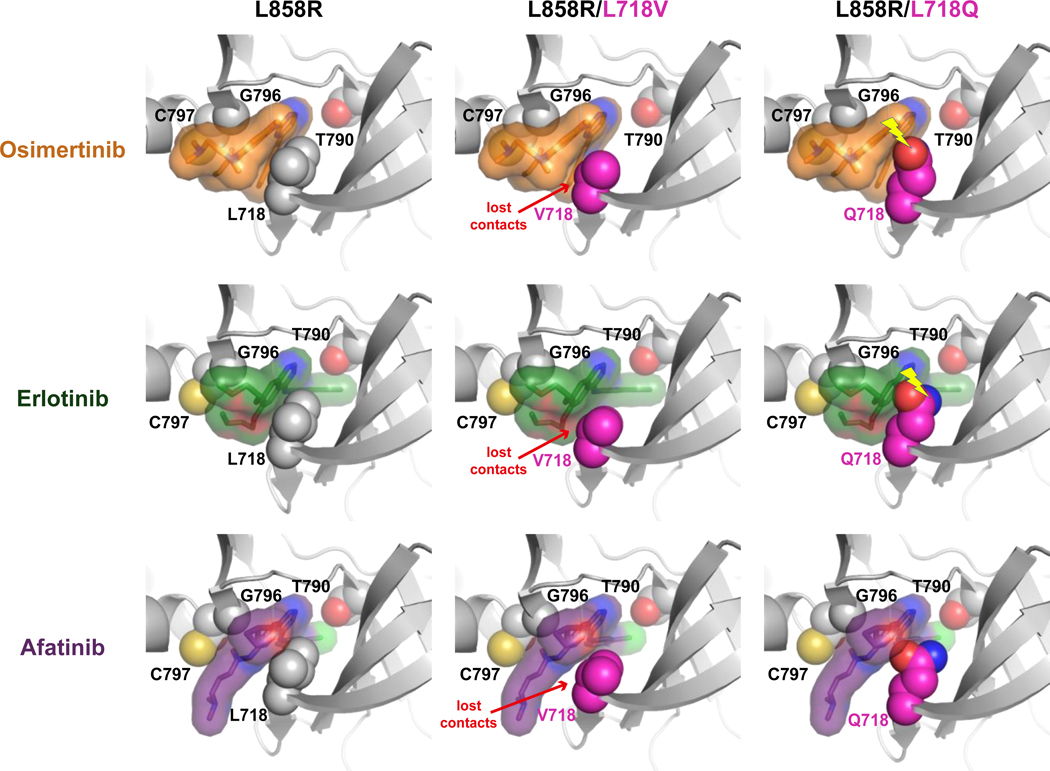
Structural models for the L858R-mutated EGFR kinase domain bound to osimertinib, erlotinib, or afatinib are shown, with (or without) the incorporation of L718V or L718Q mutations as described in the Supplementary Methods. In each case, the bound drug molecule of the energy minimized model has been replaced with that bound to the L858R TKD lacking an L718 mutation in order to show how drug binding is impacted. Interactions with drug are lost in all L718V variants (and drug is reoriented slightly to compensate). Clashes between the Q718 side-chain and drug (denoted by yellow lightning bolt) are seen for the osimertinib and erlotinib complexes (with resulting drug reorientation), but not for afatinib.
