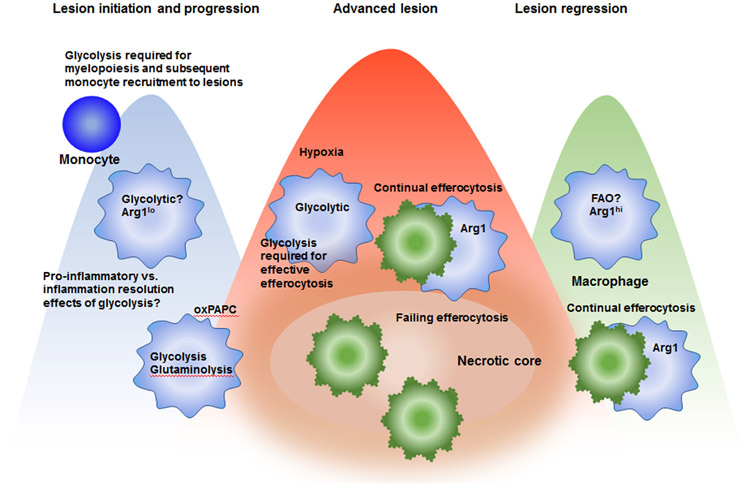
Figure 2. Schematic representation of some of the likely immunometabolic phenotypes of lesional macrophages in different phases of atherosclerosis.
In early lesions and progressing lesions, macrophages are likely activated to exhibit a pro-inflammatory phenotype, characterized by aerobic glycolysis and low Arg1 expression. This glycolytic phenotype is required for optimal production of cytokines and chemokines and also for termination of the inflammatory response. The relative importance of proinflammatory versus inflammation resolution effects of increased glycolysis in lesions is unknown. Glycolysis is also required for myelopoiesis in the bone marrow, which indirectly affects the growing lesion as it allows an increased accumulation of blood monocytes. Lesional macrophages are exposed to increased levels of oxidized phospholipids (oxPAPC), which result in a metabolic shift characterized by simultaneous glycolysis and mitochondrial respiration, and glutaminolysis and oxaloacetate accumulation, leading to IL-1β production. As an advanced lesion is formed, hypoxic regions are likely present at a greater extent, which further enhances macrophage aerobic glycolysis. At the same time, continual efferocytosis becomes overwhelmed and macrophage death predominates over clearance of dead cells. In regressing lesions, macrophages take on an alternative activated phenotype, characterized by increased Arg1 expression and which is likely also characterized by increased fatty acid oxidation (FAO) vis-à-vis glycolysis. Together, these processes would be predicted to increase efferocytosis efficiency and reduce inflammatory processes.