Abstract
Objective
The root knot nematodes (RKN) Meloidogyne incognita can severely reduce grapevine yields over time. Grapevine rootstocks have been developed from wild Vitis species that provide resistance to nematode infections. However, the potential biochemical or mechanical mechanisms of resistance have not been thoroughly explored. Therefore, this study measured levels of stilbenoids in roots of non-infected and RKN-infected grapevines with Cabernet Sauvignon scion grafted to susceptible (O39-16) or resistant (Freedom) rootstocks. This was part of a larger effort to assess phenolic compound levels within grapevine rootstocks to determine roles of stilbenoid compounds in improving nematode resistance and overall plant health.
Results
None of the assessed compounds were consistently greater in RKN infected plants versus healthy controls. Stilbenoids putatively identified as pallidol, ɑ-viniferin, miyabenol C, and hopeaphenol were overall much greater in Freedom than O39-16 rootstocks. By contrast, the stilbenoids ampelopsin A, ω-viniferin, and vitisin B were greater in O39-16 than Freedom. O39-16 and Freedom had similar levels of other stilbenoids especially monomers and dimers. Potentially the greater levels of specific stilbenoids present in Freedom than O39-16 provided RKN resistance. If validated, breeding programs could utilize the increased presence of these compounds as a marker for increased resistance to nematodes.
Keywords: Induced defense responses, Phenolics, Plant host resistance, Stilbenoids, Vitis champinii, Vitis rotundifolia, Vitis riparia, Vitis vinifera
Introduction
Root knot nematodes (RKN, Meloidogyne spp.) can be major pathogens almost everywhere grapevines are grown as populations can build up over time to severely affect root functioning, with effects on overall plant health and yields [1].
Most commercial grapevines are now Vitis vinifera cultivars grown as scions grafted onto rootstocks, as some of these rootstocks possess medium to high levels of resistance to RKN from breeding projects dating back to the 1950s [2–8]. The mechanisms for resistance to RKN remain unclear, with work to characterize how grapevines could ward off nematodes only beginning [4]. One potential mechanism is the production of a class of phenolic compounds called stilbenoids, which are mostly associated with being antibiotics against microbes [9]. A recent study of stilbenoids present in roots of grapevine was conducted on self-rooted ‘Cabernet Sauvignon’ and quantified high levels of five stilbenoid compounds: resveratrol, piceatannol, piceid, ε-vinifierin, and δ-viniferin [10].
However, more stilbenoids exist in grapevine rootstocks as multiple species can comprise specific ones. This is the first study to relate concentrations of stilbenoids to observed resistance that grapevine rootstocks may possess against nematodes. Thus, stilbenoid levels were assessed in a susceptible rootstock cultivar ‘O39-16′ (V. vinifera × Vitis rotundifolia) and a resistant rootstock cultivar ‘Freedom’ [Vitis champinii × (Vitis solonis x (V. vinifera × (Vitis riparia × V. labrusca)))]. Future and ongoing studies will examine additional rootstocks with different backgrounds. Findings could be used to aid the development of novel RKN resistance molecular markers for use in grapevine breeding efforts.
Main text
Materials and methods
Experimental design and sample collection
In both June of 2015 and 2016, a total of 16 for each of 2 year old ‘Cabernet Sauvignon’ grapevines either grafted to ‘O39-16′ (RKN-susceptible) or ‘Freedom’ (RKN-resistant) [7, 13] in 3 gallon pots were inoculated with a RKN, Meloidogyne incognita (Kofoid & White) Chitwood, by pipetting 10 mL of a nematode suspension, containing a total of 1000 nematode eggs, into the soil around the plants. The treatments were arranged as a completely randomized block design, with the plants kept in temperature-controlled greenhouse (about 22 °C to 32 °C), carefully watered weekly to avoid water flow-through, and received natural sunlight for the entire duration of the experiment. Four controls and four RKN infected plants were harvested at 6 and 12 weeks post-inoculation treatment. At each harvest, the plants were removed from the pots, with the roots briefly rinsed in water, and sampled by using pruning shears to collect six semi-randomly collected segments covering fine, lateral, and tap roots (roughly 10 g total were collected) for nematode extractions, and additional roots were collected similarly and flash-frozen in liquid nitrogen and kept at − 20 °C for compound extractions. The leftover soil was then hand-mixed with roughly 50 cm3 collected in a 50 mL centrifuge tube for soil nematode counts.
Root knot nematode counts
RKN counts were made in both the root tissues and collected soil. In brief, modified Baermann funnels were set up with filter paper, on which a weighed amount of roots were (roughly 5 g) submerged in water. The end of the funnel had a small amount of rubber tubing closed with a binder clip. Likewise, 50 cm3 of soil was measured out and placed on filter paper and submerged. After 48 h, the water was collected from the funnel assembly, and brought to 10 mL total. A 1 mL aliquot of this was then placed in a deep-well microscope slide with a 4 1 × 1 mm grid for counting RKN at the mobile juvenile (J2) stage. Final counts were adjusted to a per g root or per cm3 soil amounts.
Stilbenoid extraction and quantification
Chemical analyses proceeded based on modified methods of Wallis et al. [11] and Wallis and Chen [12]. All reagents and solvents were provided by Thermo-Fisher Scientific (Waltham, MA, USA). All frozen root samples, including some with galls, were pulverized with a mortar and pestle in liquid nitrogen and had three 0.10 g aliquots weighed out into three 1.5 mL centrifuge tubes and then extracted overnight at 4 °C in methanol. Remaining pellets were re-extracted in 0.5 mL of the same solvent, with this second extract combined with the first 1.0 mL total extract after combination.
High-performance liquid chromatography (HPLC) was used to examine stilbenoid compounds from these methanol extracts. A total of 50 µL of the methanol extract was injected into a Shimadzu (Columbia, MD, USA) LC-20AD pump based liquid chromatograph equipped with Supelco Ascentis RP-18 (Sigma-Aldrich, St. Louis, MO, USA) column and a Shimadzu PDA-20 photodiode array detector. Sigma-Aldrich provided piceatannol, resveratrol, and ε-viniferin, which were used to identify these compounds. Other compounds were identified via liquid chromatography-mass spectrometry using a Shimadzu LCMS2020 system [12] and comparing molecular weight information and relative retention times with those previously reported for grapevine stems and roots (Table 1). The obtained weights of phenolics present within samples were derived by running standard curves made using resveratrol [12].
Table 1.
Compounds quantified in this study and criteria used for putative identifications
| Stilbenoid type | Putative name | Retention time | Molecular weight | References |
|---|---|---|---|---|
| Monomer | Piceatannol | 19.1 | 243 | [14–16] |
| Resveratrol | 21.5 | 228 | [14–16] | |
| Dimer | Ampelopsin A | 16.3 | 469 | [14, 15] |
| Ampelopsin D/ quadrangularin A | 18.8 | 454 | [17] | |
| ε-viniferina | 24.0 | 454 | [10, 14–17] | |
| Pallidol | 18.1 | 454 | [17] | |
| ω-viniferin | 26.6 | 454 | [15, 16] | |
| Trimer | α-viniferin | 24.7 | 680 | [17] |
| Miyabenol C | 22.3 | 680 | [14, 15, 17] | |
| Tetramer | Hopeaphenolb | 25.9 | 906 | [14, 15] |
| Vitisin B | 27.8 | 906 | [15, 16] |
aCompound potentially co-eluted with δ-viniferin
bCompound potentially co-eluted with isohopeaphenol
Statistical analyses
IBM (Armonk, NY, USA) SPSS statistics version 22, with α = 0.05, was used for all statistical analyses. Outliers consistently greater than two standard errors of a mean for each factor were excluded from analyses [11]. Unless stated, for all analyses N = 32.
Due to a lack of meeting normality assumptions, non-parametric Mann–Whitney U tests were used to confirm differences in RKN nematodes present within soil and roots among the grapevine rootstock cultivars (O39-16 or Freedom), for each sampling time (6 or 12 weeks), and for each year (2015 or 2016) for RKN-inoculated plants only, as all non-inoculated plants did not have RKN observed.
Analyses of variance were employed to compare differences in individual compounds between rootstock cultivars and differences due to inoculation status (with the interaction also in the model). Each year was treated as a separate experimental trial.
Results and discussion
Nematode counts
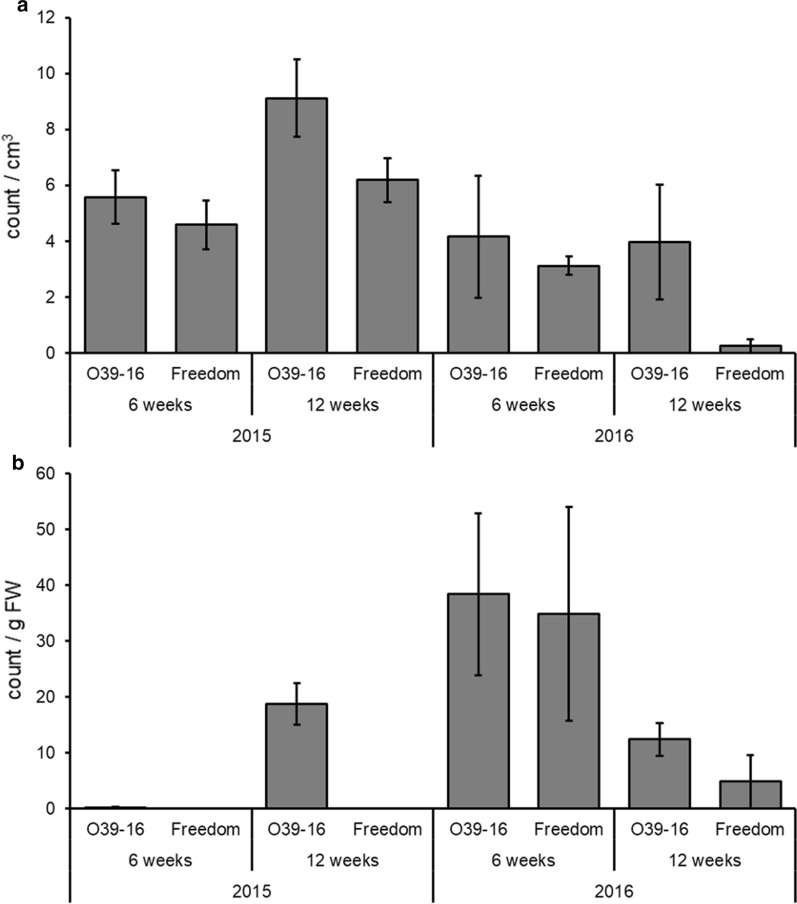
Nematode levels, measured as J2 stage juveniles, in the soil were significantly less when the resistant ‘Freedom’ was used instead of susceptible ‘O39-16′ as the rootstock (Mann–Whitney U = 73.000; P = 0.038) (Fig. 1a). Fewer nematodes were counted in the soil in 2015 than 2016 (Mann–Whitney U = 50.500; P = 0.003). There were no differences in soil nematode counts between sampling done in week 6 compared with week 12 (Mann–Whitney U = 104.500; P = 0.375).
Fig. 1.
Mean nematode (J2 stage) counts (± SE) present in (a) soil or (b) roots of inoculated plants. FW fresh weight
Nematode counts in the roots, measured as J2 juveniles collected via Baermann funnels, were greater in the susceptible ‘O39-16′ rootstocks than resistant ‘Freedom’ (Mann–Whitney U = 60.500; P = 0.009) (Fig. 1b). Nematode counts in root samples also were less in 2015 than 2016 (Mann–Whitney U = 62.500; P = 0.011). There was no a significant difference in root nematode counts when samples were collected at 6 versus 12 weeks (Mann–Whitney U = 113.500; P = 0.574).
Potential differences in nematode growth rates, due to variances in environmental conditions and pre-existing plant health, likely resulted in different counts between weeks and years. Future studies are warranted whereby nematode populations are more carefully measured over an increased time course, such as weekly or biweekly for several months, to appropriately assess fluxes in nematode counts over time.
However, these results likely confirm previous observations about resistance, as it was observed that ‘Freedom’ rootstocks possess some resistance to nematode infections whereas ‘O39-16′ rootstocks were susceptible [7, 13].
Root stilbenoid levels
A total of eleven stilbenoids were putatively identified in this study, and each compound was analyzed by week and year separately (Table 1). The stilbenoid compounds quantified in this study were similar as those found in other studies (Table 1), albeit the resveratrol glycoside piceid was not observed in quantifiable amounts, with only trace characteristic ions observed by LC–MS, despite being observed previously [10, 14]. However, piceid also was not present in sufficiently quantifiable amounts in V. vinifera by Lambert et al. [15] or in many of the wild Vitis spp. studied by Pawlus et al. [16]. The putatively identified miyabenol C, hopeaphenol, and ε-viniferin were the most prevalent stilbenoids observed in this study, which was like previous observations [10, 14, 15].
For most analyses, there were no significant differences due to infection status, with a few exceptions (Table 2). Nematode infections increased levels of piceatannol, ampelosin D/quandrangularin A, and ɑ-viniferin in week 12 of 2016. By contrast, ampelopsin A, ω-viniferin, and vitisin B were present in lower levels in nematode infected plants compared to controls during week 12 of 2016. Pallidol had greater levels in nematode infected plants compared to controls in week 6 of 2016. Despite inherit variability in this study in terms of nematode populations, the findings that certain stilbenoid compounds increased suggests some induction of these compounds occurred as a host response. As for the lack of other compounds from being affected by feeding, it could be hypothesized that RKN manipulations of host cells altered compound levels, albeit unevenly in the root samples of this study as both galled and ungalled tissues were analyzed. Further, RKN might possess mechanisms that reduced or altered host responses associated with herbivory.
Table 2.
Mean (± SE) concentrations of individual stilbenoids (µg/g FW) in healthy or RKN-infected roots
| O39-16 | Freedom | F-statistic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stilbenoid type | Putative name | Year | Week | Control | RKN | Control | RKN | Cultivar | Inoculation | Interaction |
| Monomer | Piceatannol | 2015 | 6 | 17.6 ± 6.5 | 37.3 ± 17.0 | 12.5 ± 3.6 | 19.0 ± 4.0 | 1.521 | 1.912 | 0.490 |
| 12 | 15.5 ± 2.7 | 75.6 ± 27.8 | 11.9 ± 2.9 | 17.6 ± 0.8 | 4.839* | 5.502* | 3.756 | |||
| 2016 | 6 | 17.5 ± 4.0 | 35.0 ± 8.5 | 36.7 ± 6.0 | 30.2 ± 18.5 | 1.588 | 0.954 | 4.509 | ||
| 12 | 26.4 ± 3.0 | 36.2 ± 11.8 | 31.8 ± 1.1 | 39.9 ± 3.7 | 0.337 | 1.709 | 0.001 | |||
| Resveratrol | 2015 | 6 | 301 ± 99 | 1170 ± 660 | 105 ± 29 | 131 ± 43 | 3.383 | 1.770 | 1.567 | |
| 12 | 100 ± 27 | 738 ± 486 | 106 ± 13 | 111 ± 40 | 1.625 | 1.737 | 1.686 | |||
| 2016 | 6 | 216 ± 27 | 323 ± 151 | 299 ± 79 | 192 ± 22 | 0.078 | 0.000 | 1.520 | ||
| 12 | 281 ± 88 | 490 ± 226 | 204 ± 25 | 252 ± 14 | 1.659 | 1.112 | 0.434 | |||
| Dimer | Ampelopsin A | 2015 | 6 | 250 ± 51 | 265 ± 62 | 204 ± 44 | 281 ± 72 | 0.055 | 0.512 | 0.241 |
| 12 | 255 ± 51 | 314 ± 67 | 235 ± 28 | 228 ± 28 | 1.211 | 0.293 | 0.469 | |||
| 2016 | 6 | 451 ± 59 | 480 ± 45 | 408 ± 77 | 304 ± 36 | 3.804 | 0.449 | 1.393 | ||
| 12 | 578 ± 47 | 307 ± 22 | 288 ± 26 | 324 ± 29 | 17.527** | 13.049** | 22.154*** | |||
| Ampelopsin D /quadrangularin A | 2015 | 6 | 11.9 ± 3.8 | 24.6 ± 9.5 | 21.8 ± 4.6 | 27.8 ± 4.3 | 1.206 | 2.435 | 0.309 | |
| 12 | 10.2 ± 1.8 | 38.3 ± 13.0 | 23.3 ± 1.7 | 26.1 ± 1.4 | 0.005 | 5.356* | 3.620 | |||
| 2016 | 6 | 26.7 ± 7.5 | 32.0 ± 9.8 | 51.1 ± 3.7 | 51.7 ± 1.3 | 11.509** | 0.209 | 0.133 | ||
| 12 | 28.3 ± 5.4 | 30.0 ± 6.3 | 52.1 ± 6.9 | 58.6 ± 17.6 | 6.430* | 0.156 | 0.054 | |||
| ε-viniferin | 2015 | 6 | 350 ± 33 | 212 ± 88 | 218 ± 63 | 174 ± 56 | 1.833 | 2.092 | 0.567 | |
| 12 | 267 ± 91 | 223 ± 23 | 131 ± 9 | 181 ± 40 | 3.024 | 0.004 | 0.836 | |||
| 2016 | 6 | 387 ± 55 | 372 ± 47 | 274 ± 49 | 234 ± 27 | 7.544* | 0.360 | 0.076 | ||
| 12 | 379 ± 9 | 214 ± 51 | 248 ± 18 | 331 ± 73 | 0.022 | 0.828 | 7.445* | |||
| Pallidol | 2015 | 6 | 69.5 ± 10.5 | 84.6 ± 20.7 | 182 ± 6 | 184 ± 40 | 20.386*** | 0.135 | 0.077 | |
| 12 | 61.3 ± 10.2 | 80.5 ± 18.4 | 141 ± 19 | 177 ± 32 | 17.415*** | 1.737 | 0.165 | |||
| 2016 | 6 | 86.0 ± 18.6 | 127 ± 15 | 122 ± 13 | 149 ± 6 | 4.433 | 5.890* | 0.255 | ||
| 12 | 125 ± 18 | 104 ± 17 | 162 ± 20 | 197 ± 43 | 6.069* | 0.070 | 1.112 | |||
| ω-viniferin | 2015 | 6 | 247 ± 49 | 208 ± 26 | 83.4 ± 16.6 | 97.0 ± 21.7 | 20.006*** | 0.175 | 0.739 | |
| 12 | 358 ± 30 | 304 ± 43 | 103 ± 9 | 105 ± 13 | 68.739*** | 0.930 | 1.029 | |||
| 2016 | 6 | 363 ± 51 | 459 ± 28 | 298 ± 75 | 204 ± 16 | 11.045** | 0.001 | 3.911 | ||
| 12 | 528 ± 46 | 312 ± 17 | 196 ± 14 | 241 ± 12 | 57.218*** | 10.148** | 23.936*** | |||
| Trimer | α-viniferin | 2015 | 6 | 33.1 ± 2.4 | 27.9 ± 5.6 | 32.7 ± 5.8 | 36.8 ± 8.1 | 0.532 | 0.008 | 0.631 |
| 12 | 41.2 ± 5.8 | 42.1 ± 5.1 | 62.9 ± 2.9 | 63.4 ± 4.2 | 21.407*** | 0.026 | 0.003 | |||
| 2016 | 6 | 78.8 ± 5.5 | 63.2 ± 2.9 | 82.6 ± 6.9 | 99.5 ± 16.2 | 4.615 | 0.005 | 3.036 | ||
| 12 | 53.3 ± 2.4 | 61.2 ± 5.5 | 74.5 ± 6.5 | 96.1 ± 5.5 | 28.269*** | 7.844* | 1.661 | |||
| Miyabenol C | 2015 | 6 | 6.39 ± 1.42 | 11.2 ± 7.4 | 148 ± 22 | 169 ± 24 | 53.106*** | 0.411 | 0.165 | |
| 12 | 6.62 ± 0.90 | 19.2 ± 4.9 | 187 ± 9 | 189 ± 29 | 101.094*** | 0.171 | 0.096 | |||
| 2016 | 6 | 69.8 ± 52.5 | 11.1 ± 2.8 | 190 ± 60 | 219 ± 18 | 16.031** | 0.128 | 1.155 | ||
| 12 | 8.67 ± 1.30 | 11.3 ± 2.1 | 216 ± 15 | 235 ± 1 | 805.294*** | 1.923 | 1.094 | |||
| Tetramer | Hopeaphenol | 2015 | 6 | 246 ± 45 | 176 ± 19 | 875 ± 224 | 1032 ± 300 | 15.514** | 0.053 | 0.362 |
| 12 | 352 ± 36 | 266 ± 41 | 884 ± 112 | 879 ± 167 | 30.134*** | 0.189 | 0.149 | |||
| 2016 | 6 | 596 ± 207 | 439 ± 19 | 1100 ± 230 | 1260 ± 120 | 16.355** | 0.000 | 0.913 | ||
| 12 | 423 ± 39 | 282 ± 20 | 1140 ± 70 | 1290 ± 30 | 388.179*** | 0.159 | 16.672** | |||
| Vitisin B | 2015 | 6 | 81.6 ± 17.3 | 69.7 ± 13.1 | 8.79 ± 1.36 | 9.70 ± 2.08 | 50.634*** | 0.345 | 0.468 | |
| 12 | 131 ± 11 | 104 ± 18 | 9.81 ± 1.12 | 12.2 ± 1.9 | 104.258*** | 1.334 | 1.910 | |||
| 2016 | 6 | 113 ± 32 | 162 ± 10 | 57.0 ± 38.1 | 17.3 ± 2.7 | 15.611** | 0.033 | 3.044 | ||
| 12 | 185 ± 19 | 105 ± 5 | 17.2 ± 1.4 | 20.1 ± 1.3 | 167.010*** | 15.505** | 17.961** | |||
*P < 0.05; **P < 0.01; ***P < 0.001
The susceptible ‘O39-16′ rootstocks consistently possessed greater levels of ω-viniferin and vitisin B than the resistant ‘Freedom’ rootstock (Table 2). By contrast, ‘Freedom’ rootstocks consistently possessed greater levels of miyabenol C and hopeaphenol (Table 2). Previously, Lambert et al. [15] observed vast differences in stilbenoid concentration among many V. vinifera cultivars, including the presence or virtual absence of certain compounds such as miyabenol C and vitisin B. Furthermore, Pawlus et al. [16] observed differences and presence or absence of certain stilbenoids among wild Vitis spp. as well. Unlike this study, Pawlus et al. [16] did not examine currently available commercial rootstock cultivars. Furthermore, although Lambert et al. [15] and Pawlus et al. [16] observed chemistry of stem tissues, this study determined similar differences when comparing stilbenoid levels in the roots of different species-derived rootstocks, namely large differences in certain specific compounds.
Conclusions
Based on these observations, a hypothesis can be formed that miyabenol C and hopeaphenol levels potential impart resistance to RKN, as these compounds were present in levels four- to ten-fold greater in resistant ‘Freedom’ rootstocks than ‘O39-16′. By contrast, it seems that many stilbenoid monomers and dimers are not involved in RKN resistance, as otherwise greater levels of dimers (such as ε-viniferin and ω-viniferin) would make ‘O39-16′ more resistant. It could be hypothesized that ‘Freedom’ possesses enzymes that were more effective at producing stilbenoid trimers and tetramers than ‘O39-16′. Targeting genes responsible for producing stilbenoid polymer synthases could reveal genetic differences between the two cultivars and may be mapped as molecular markers of RKN resistance. These new markers could prove valuable in breeding efforts to impart RKN resistance in newly developed rootstocks.
Limitations
This data set is limited by including only two cultivars, just one resistant and one susceptible, so firm conclusions about the roles of stilbenoids cannot be made at this time. Additional studies across a broader spectrum of both RKN susceptible and resistant rootstocks, and possible crosses between these rootstocks, would be necessary to support conclusions. Likewise, this study likely did not use a large enough inoculum to conduct this experiment- future studies should be inoculated with contaminated soil or roots to provide a greater RKN population and a variety of different life stages. Furthermore, sampling should be at an increased interval in future studies to capture fluctuations in RKN populations over time, perhaps incorporating weekly or biweekly sampling. Assessment of nematodes also should include gall counts/disease assessments as well to provide a more accurate picture of effects on host health. Other compounds and defense proteins also are likely involved in host defense against RKN. There also is the possibility that nutritional differences or unmeasured effects on overall plant health that differ between rootstock cultivars also could result in observed differences in RKN susceptibility. Lastly, bioassays that directly or indirectly observe the effects of stilbenoids on nematode reproduction, feeding, or survival would be necessary to support the hypotheses that certain compounds impart resistance. Unfortunately, the major of stilbenoid compounds are not commercially available, and time-consuming isolations or syntheses are needed for these studies to proceed.
Acknowledgements
The author thanks Nancy Goodell, Julie Pedraza, Mala To, and Justin King for their technical assistance in this work. The author also thanks Andreas Westphal from the University of California, Parlier, CA, for providing root knot nematodes and expertise in counting and inoculation used to perform these studies. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Abbreviation
- RKN
Root knot nematode
Author contributions
CMW conducted all aspects of this work. The author read and approved the final manuscript.
Funding
The work was funded by allocated funds to the San Joaquin Valley Agricultural Sciences Center, U.S. Department of Agriculture.
Availability of data and materials
The data described in this Data note can be freely and openly accessed on the USDA Ag Data Common (https://data.nal.usda.gov/dataset/grapevine-rootstock-stilbenoid-data-and-rkn-induction).
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McKenry MV. Nematodes. In: Flaherty DL, Christensen LP, Lanini WT, Marois JJ, Phillips PA, Wilson LT, editors. Grape pest management. Oakland: University of California; 1992. pp. 279–296. [Google Scholar]
- 2.Lider LA. Vineyard trials in California with nematode resistant grape rootstock. Hilgardia. 1960;30:123–152. doi: 10.3733/hilg.v30n04p123. [DOI] [Google Scholar]
- 3.Walker MA, Ferris H, Eyre M. Resistance in Vitis and Muscadinia species to Meloidogyne incognita. Plant Dis. 1994;78:1055–1058. doi: 10.1094/PD-78-1055. [DOI] [Google Scholar]
- 4.Saucet SB, van Ghelder C, Abad P, Duval H, Esmenjaud D. Resistance to root-knot nematodes Meloidogyne spp. in woody plants. New Phytol. 2016;211:41–56. doi: 10.1111/nph.13933. [DOI] [PubMed] [Google Scholar]
- 5.Smith HM, Smith BP, Morales NB, Moskwa S, Clingeleffer PR, Thomas MR. SNP markers tightly linked to root knot nematode resistance in grapevine (Vitis cinerea) identified by a genotype-by-sequencing approach followed by Sequenom MassARRAY validation. PLoS ONE. 2018;13:e0193121. doi: 10.1371/journal.pone.0193121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anwar SA, McKenry MV. Penetration and development of Meloidogyne arenaria on two new grape rootstocks. J Nematol. 2002;34:143–145. [PMC free article] [PubMed] [Google Scholar]
- 7.Esmenjaud D, Bouquet A. Selection and application of resistant germplasm for grapevine nematodes management. In: Ciancio A, Mukerji KG, editors. Integrated management of fruit crops and forest nematodes. Heidelberg: Springer Science BV; 2009. pp. 195–214. [Google Scholar]
- 8.Ferris H, Zheng L, Walker MA. Resistance of grape rootstocks to plant-parasitic nematodes. J Nematol. 2012;44:377–386. [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon RA. Natural products and plant disease resistance. Nature. 2001;411:843–847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- 10.Wei Y-J, Zhao S-R, Li J-M, Xue B. Stilbene profiles in different tissues of Vitis vinifera L. cv. Cabernet Sauignon and a comparison of their antioxidant activity. Austral J Grape Wine Res. 2016;22:226–231. doi: 10.1111/ajgw.12230. [DOI] [Google Scholar]
- 11.Wallis C, Eyles A, McSpadden Gardener B, Hansen R, Cipollini D, Herms DA, Bonello P. Systemic induction of phloem secondary metabolism and its relationship to resistance to a canker pathogen in Austrian pine. New Phytol. 2008;177:767–778. doi: 10.1111/j.1469-8137.2007.02307.x. [DOI] [PubMed] [Google Scholar]
- 12.Wallis CM, Chen J. Grapevine phenolic compounds in xylem sap and tissues are significantly altered during infection by Xylella fastidiosa. Phytopathol. 2012;102:816–826. doi: 10.1094/PHYTO-04-12-0074-R. [DOI] [PubMed] [Google Scholar]
- 13.Keller M. The science of grapevines: anatomy and physiology. 1. San Francisco: Academic Press (Elsevier); 2010. [Google Scholar]
- 14.Lambert C, Bisson J, Waffo-Téguo P, Papastamoulis Y, Richard T, Corio-Costet M-F, Mérillon JM, Cluzet S. Phenolics and their antifungal role in grapevine wood decay: focus on the Botryosphaeriaceae family. J Agri Food Chem. 2012;60:11859–11868. doi: 10.1021/jf303290g. [DOI] [PubMed] [Google Scholar]
- 15.Lambert C, Richard T, Renouf E, Bisson J, Waffo-Téguo P, Bordenave L, Ollat N, Mérillon JM, Cluzet S. Comparative analyses of stilbenoids in canes of major Vitis vinifera L. cultivars. J Agri Food Chem. 2013;61:11392–11399. doi: 10.1021/jf403716y. [DOI] [PubMed] [Google Scholar]
- 16.Pawlus AD, Sahli R, Bisson J, Riviere C, Delaunay J-C, Richard T, Gomes E, Bordenave L, Waffo-Téguo P, Mérillon JM. Stilbenoid profiles of canes from Vitis and Muscadinia species. J Agri Food Chem. 2013;61:501–511. doi: 10.1021/jf303843z. [DOI] [PubMed] [Google Scholar]
- 17.Mattivi F, Vrhovsek U, Malacarne G, Masuero D, Zulini L, Stefanini M, Moser C, Velasco R, Guella G. Profiling of resveratrol oligomers, important stress metabolites, accumulating in the leaves of hybrid Vitis vinifera (Merzling × Teroldego) genotypes infected with Plasmopara viticola. J Agri Food Chem. 2011;59:5364–5375. doi: 10.1021/jf200771y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data described in this Data note can be freely and openly accessed on the USDA Ag Data Common (https://data.nal.usda.gov/dataset/grapevine-rootstock-stilbenoid-data-and-rkn-induction).