FIG 1.
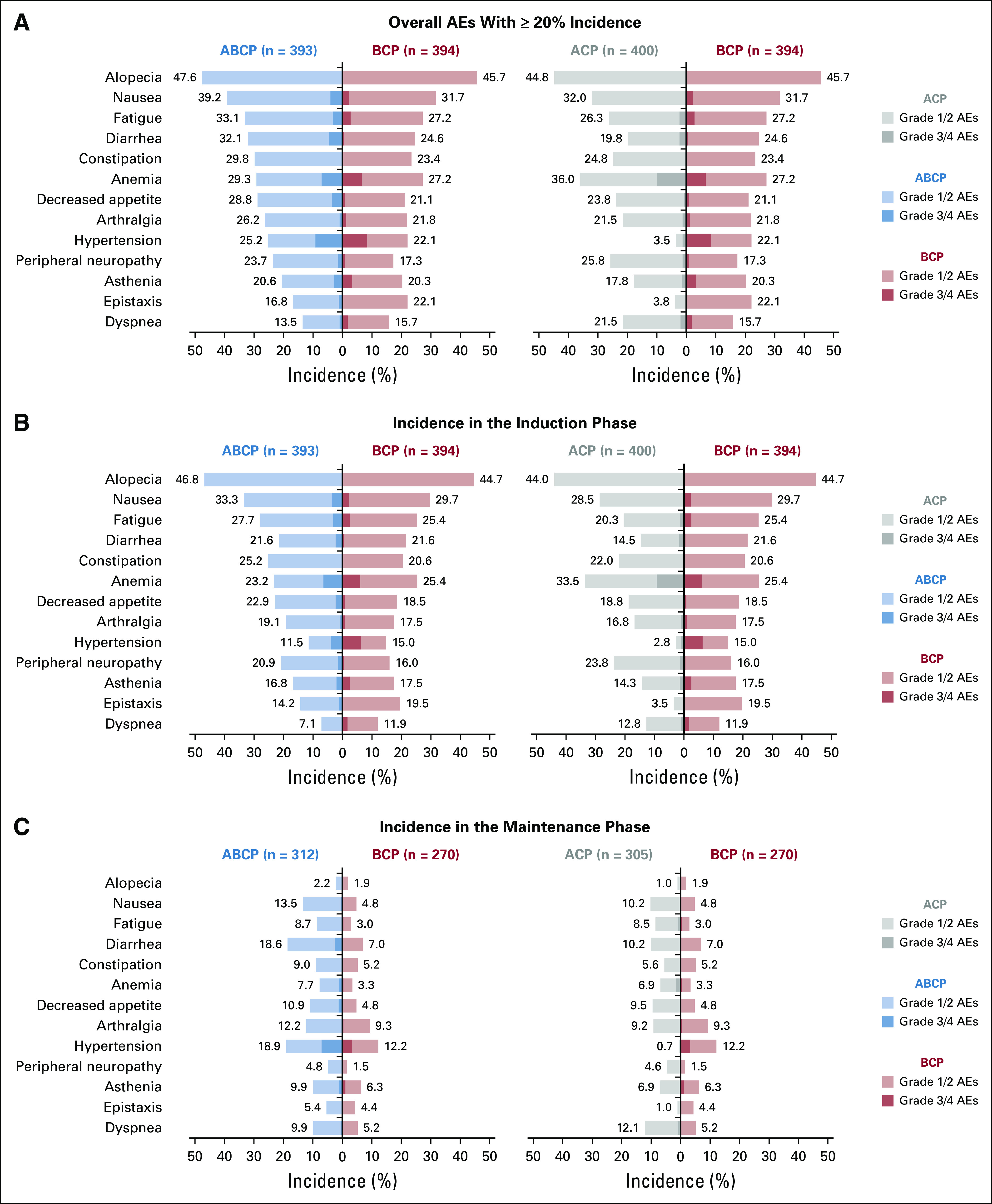
Most common adverse events (AEs) overall and by phase of treatment. (A) Incidences of the most common (≥ 20% overall incidence) AEs reported in any treatment arm. Incidences of the most common AEs in the (B) induction and (C) maintenance phases. AEs that occurred during the induction phase had an onset on or after the first study drug treatment and up to 1 day before the date of the first dose of the maintenance therapy. AEs that occurred during the maintenance phase had an onset on or after the first dose of maintenance therapy. Overall AEs represent the sum of AEs with onset during the induction phase, the maintenance phase, and/or the AE reporting period. The AE reporting period includes those patients who discontinued induction therapy, never received maintenance therapy, and had an AE with onset > 30 days after the last dose of study treatment and during the AE reporting period as defined in the protocol. ABCP, atezolizumab plus bevacizumab, carboplatin, and paclitaxel; ACP, atezolizumab, carboplatin, and paclitaxel; BCP, bevacizumab, carboplatin, and paclitaxel.
