Fig. 1. Electron affinities across the periodic table.
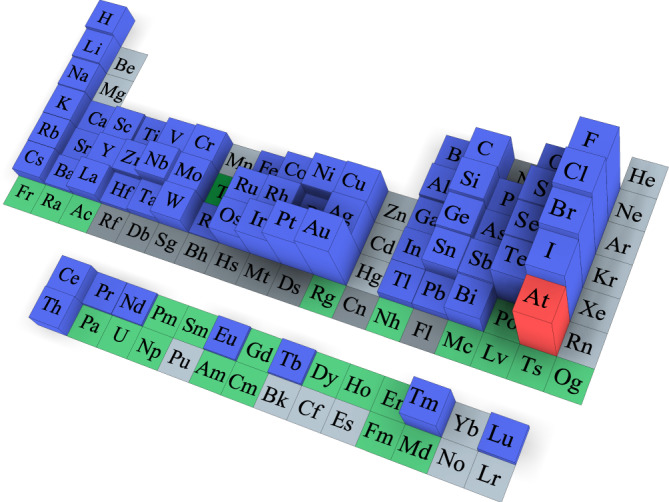
The height corresponds to the measured value of the electron affinity of the corresponding element7,8,67. Astatine is highlighted in red. Blue indicates elements that are experimentally determined to have a positive EA, i.e., to form stable negative ions. Elements that are predicted to form stable negative ions but have not yet been experimentally investigated are indicated in green, while those in light gray are predicted to not form a stable negative ion, i.e., have a negative EA. Finally, elements that neither have been experimentally observed nor investigated theoretically, are indicated with dark gray.
