FIGURE 1.
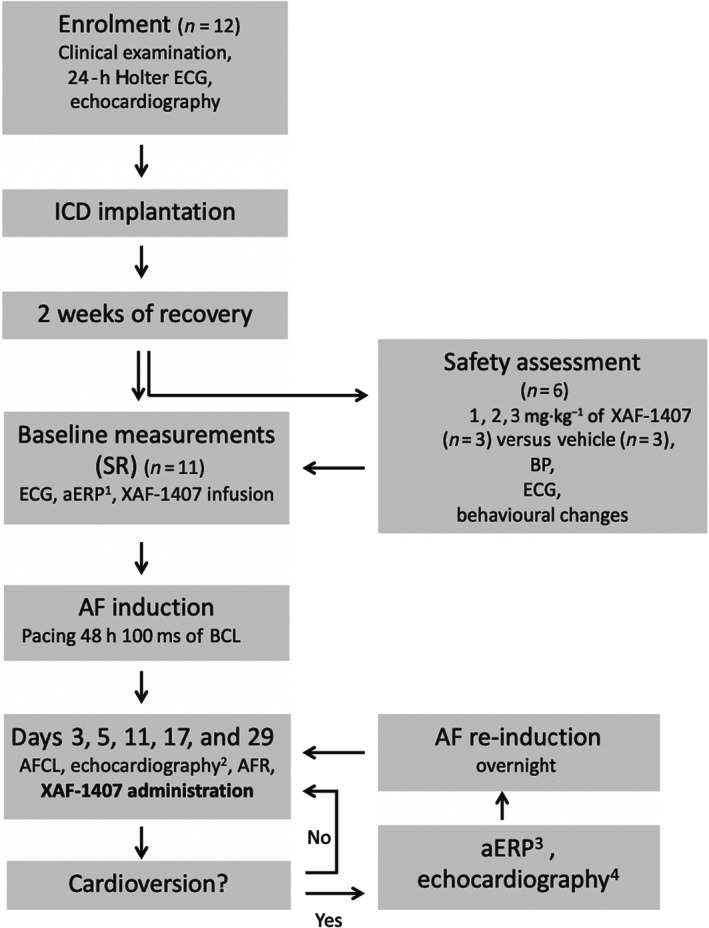
Experimental set‐up (in vivo): Following insertion of the implantable cardioverter defibrillator (ICD) and a recovery period, the safety assessment and baseline measurements were made in sinus rhythm (SR), including measurements of atrial effective refractory period (aERP) which were performed before and after XAF‐1407 infusion (at 1, 2, and 3 h). After initial induction of atrial fibrillation (AF), electrophysiological measurements were made and serial cardioversion attempts made on day 3, 5, 11, 17 and 29. If cardioversion occurred, aERP was measured 1 h after end of the XAF‐1407 infusion, before overnight re‐induction of AF. Echocardiographic examinations were conducted at enrolment (in SR), day 5 and 29 in AF, as well as in SR, if cardioversion occurred
