FIGURE 2.
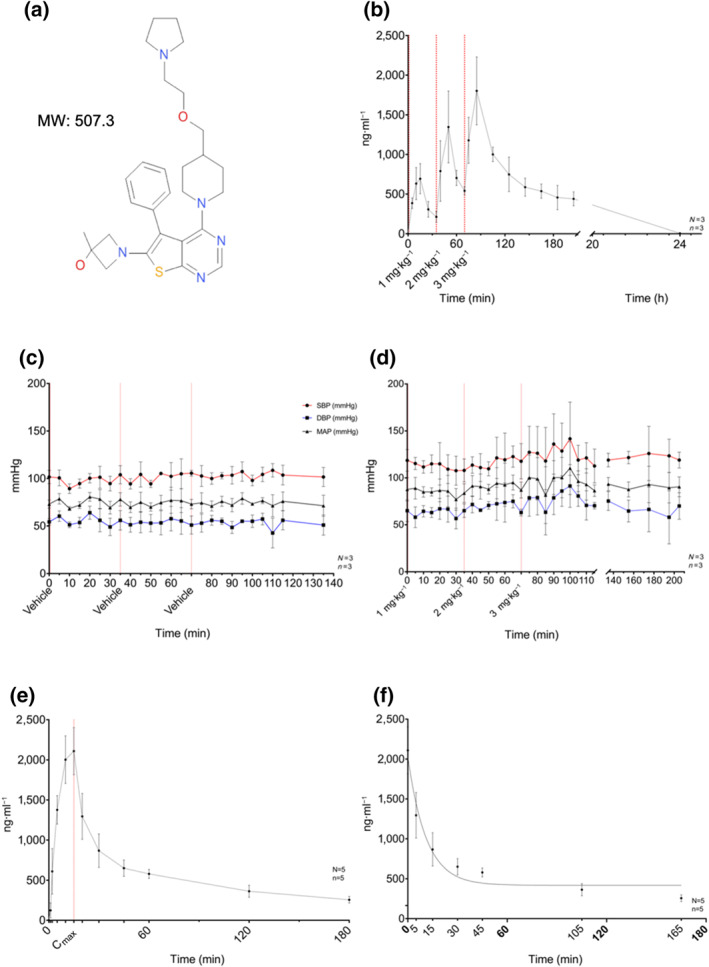
Summary of in vivo pharmacology and pharmacokinetics of XAF‐1407: (a) chemical structure and MW of 508.3 Da (XAF‐1407). (b) Plasma concentration of XAF‐1407 during the safety and tolerability assessment, measured at time points T = 0, 5, 10, 15, 25, 35, 40, 50, 60, 70, 75, 85, 105, 125, 145, 165, 185, and 205 min and 24 h in relation to start of drug administration of the first of three subsequent doses of XAF‐1407 (1, 2, and 3 mg·kg−1). (c) Non‐invasive BP monitoring during the safety and tolerability assessment for XAF‐1407 (n = 3). (d) Non‐invasive BP monitoring during the safety and tolerability assessment for vehicle (NaOAc) (n = 3). (e) Measured average plasma concentration of XAF‐1407 in equine plasma samples repeatedly from start of drug administration to 180 min after the end of the infusion. (f) Measured plasma concentration of XAF‐1407 after bolus injection of 3 mg·kg−1 XAF‐1407 fitted with an exponential 1‐phase decay model
