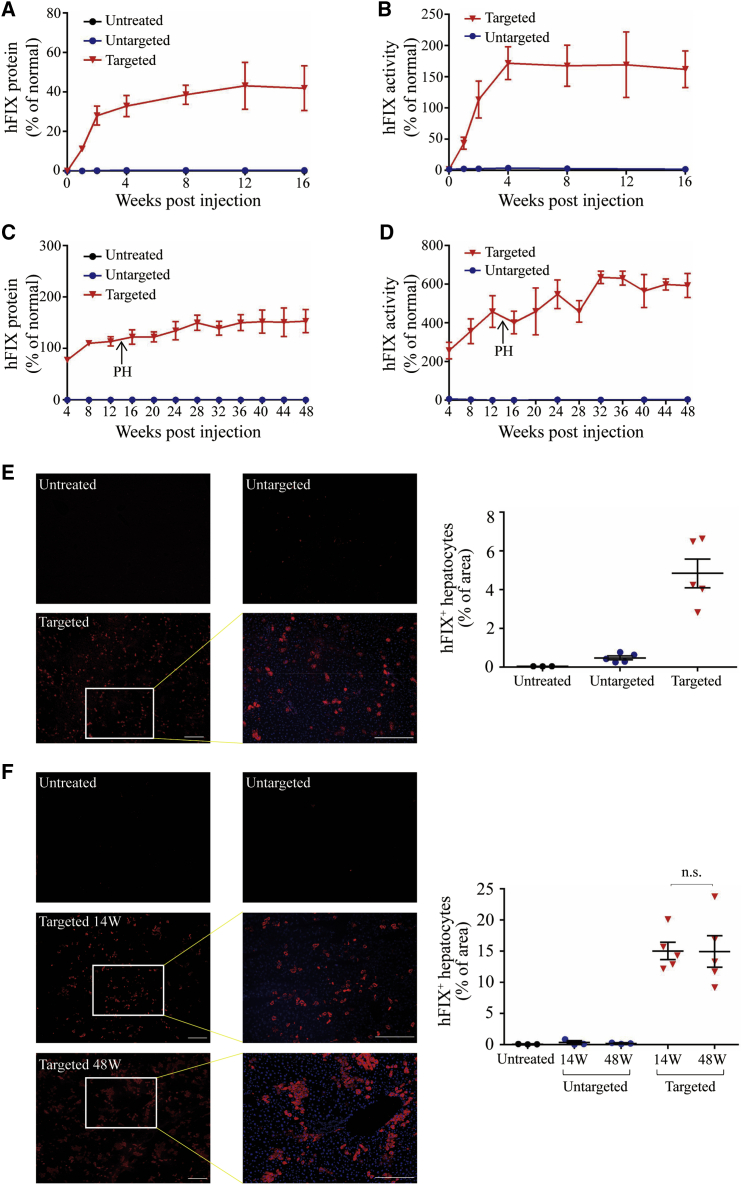
Figure 2.
Efficient Restoration of hFIX Expression in Hemophilia B Mice Treated by Dual AAV8 Strategy
(A) Plasma hFIX was measured by ELISA following tail vein injections of 8-week-old male hemophilia B mice with the AAV8.SpCas9 (9 × 1010 GC/mouse) and AAV8.sgRNA.donor (4.5 × 1011 GC/mouse) (n = 5). Untargeted hemophilia B mice received AAV8.SpCas9 (9 × 1010 GC/mouse) and AAV8.control.donor (4.5 × 1011 GC/mouse) (n = 5). Untreated hemophilia B mice (n = 5) were included as control. (B) hFIX activity in adult-treated hemophilia B mouse plasma. (C) Plasma hFIX was measured by ELISA following temporal vein injections of newborn male hemophilia B mice with the AAV8.SpCas9 (3 × 1010 GC/mouse) and AAV8.sgRNA.donor (1.5 × 1011 GC/mouse) (n = 5). All targeted mice were subjected to two-thirds partial hepatectomy (PH) 14 weeks after vector treatment. Untargeted hemophilia B mice received AAV8.SpCas9 (3 × 1010 GC/mouse) and AAV8.control.donor (1.5 × 1011 GC/mouse) (n = 6). Three untargeted mice were sacrificed at week 14 for analyses. Untreated hemophilia B mice (n = 3) were included as control. (D) hFIX activity in hemophilia B mouse plasma after neonatal vector treatment. (E) Immunofluorescence staining with antibodies against hFIX (red) with 4′,6-diamidino-2-phenylindole (DAPI) nuclear counterstain (blue) on liver sections, which were treated as adults collected at 16 weeks after injection (left). Scale bars, 100 μm. Quantification of gene expression was based on the percentage of area on liver sections expressing hFIX by immunostaining (right). (F) Immunofluorescence staining with antibodies against hFIX with DAPI nuclear counterstain on liver sections, which were treated as newborns collected at 14 and 48 weeks after injection (left). Scale bars, 100 μm. Quantification of gene expression was based on the percentage of area on liver sections expressing hFIX by immunostaining (right). Error bars represent mean ± SEM. Dunnett’s test. n.s., not significant.