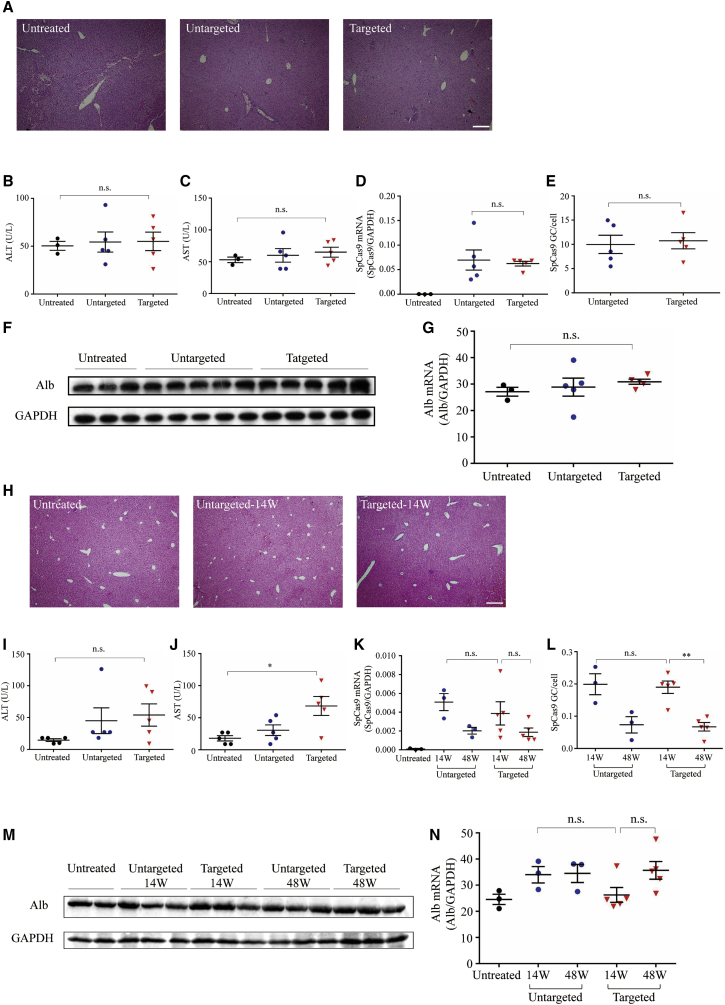
Figure 5.
Liver Function Tests and Toxicity Examination in Adult and Newborn Animals Treated with Dual AAV8 Vectors
(A) Histological analysis by hematoxylin and eosin stain on adult livers harvested 16 weeks following dual vector treatment. Scale bar, 100 μm. (B and C) Liver transaminase (B, ALT; C, AST) levels in untreated hemophilia B mice (n = 3) or 16 weeks following targeted vector (n = 5) and untargeted vector (n = 5) treatment. (D) Quantification of SpCas9 mRNA levels in liver isolated from adult mice 16 weeks after vector treatment by qPCR. (E) Quantification of SpCas9 vector genome in liver from adult treatment group by qPCR. (F) Western blot analysis. Liver lysates were prepared from adult untreated or hemophilia B mice treated with the dual AAV vectors for detection of Alb protein. (G) Quantification of Alb mRNA in the liver by qPCR. (H) Histological analysis by hematoxylin and eosin stain on newborn livers harvested 14 weeks following dual vector treatment. Scale bar, 100 μm. (I and J) ALT and AST levels in untreated hemophilia B mice (n = 5) or newbron mice 14 weeks after targeted vector (n = 5) and untargeted vector (n = 5) treatment. (K) Quantification of SpCas9 mRNA levels in liver isolated from newborn mice 14 and 48 weeks after vector treatment by qPCR. (L) Quantification of SpCas9 vector genome in liver from newborn treatment group by qPCR. (M) Western blot analysis. Liver lysates were prepared from untreated or newborn hemophilia B mice treated with the dual AAV vectors for detection of Alb protein. (N) Quantification of Alb mRNA in the liver by qPCR in newborn treatment group. Means ± SEM are shown. Dunnett’s test. ∗p < 0.05, ∗∗p < 0.01. n.s., not significant.