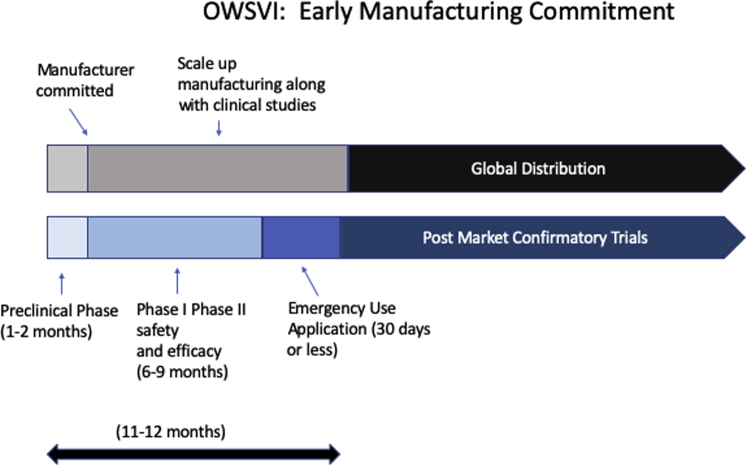
Figure 1.
OWSVI Phases With Early “All-In” Manufacturing Commitments
The manufacturer begins the process to scale up immediately after partnership commitment. Large-scale manufacturing begins during phase II, before clinical efficacy and safety are fully demonstrated, gambling that the vaccine will succeed. Emergency Use Authorization applications are submitted as soon as clinical efficacy has been demonstrated, which in this vaccine’s case will likely be determined by surrogate endpoints or biomarkers rather than by infection rates and mortality. Phase III can then be deferred to post-market clinical studies while global manufacturing and distribution proceed. OWSVI = Operation Warp Speed Vaccine Initiative.