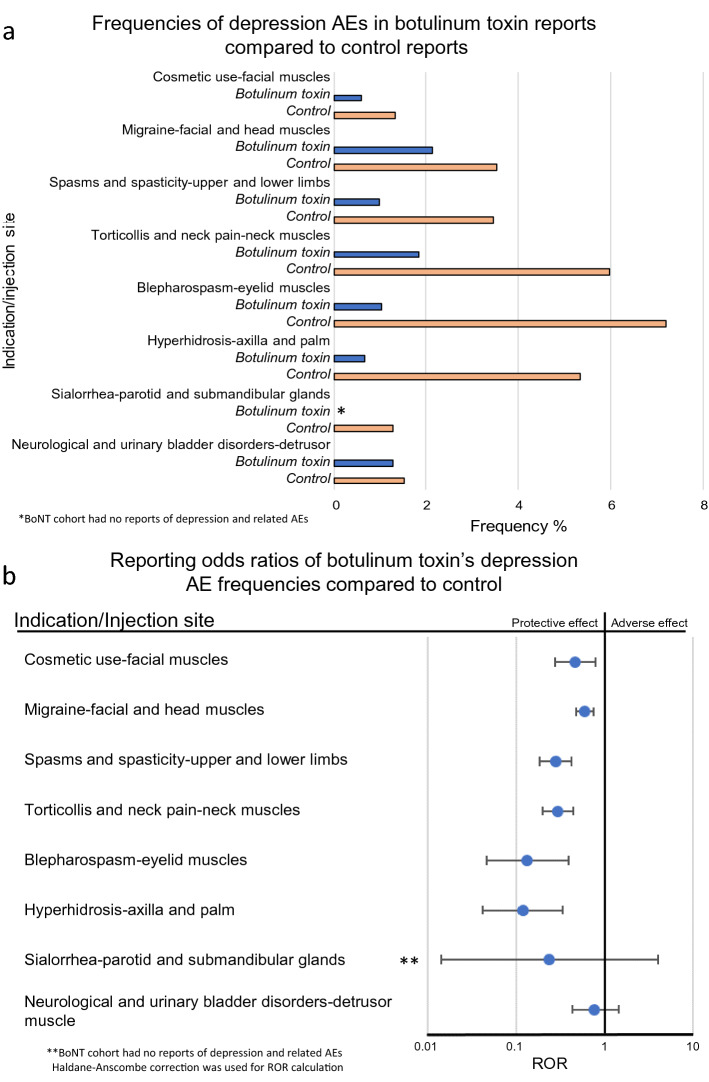
Figure 3.
Frequencies and reporting odds ratios (RORs) of depression events. (a) Frequencies of depression events for patients administered botulinum toxin for cosmetic use (BoNT N = 20,684, control N = 1,214), migraine (BoNT N = 4,180, control N = 56,675), spasms and spasticity, excluding facial muscles (BoNT N = 2,335, control N = 45,824), torticollis (BoNT N = 1,360, control N = 6,765), blepharospasm (BoNT N = 487, control N = 153), hyperhidrosis (BoNT N = 601, control N = 712), sialorrhea (BoNT N = 157, control N = 1,174), neurological and urinary bladder disorders (BoNT N = 915, control N = 30,827). (b) Reporting odds ratios were calculated comparing frequencies of depression reports in patients administered botulinum toxin for each indication and respective control sub-cohorts. Ranges represent 95% confidence intervals (95% CI) (see “Methods”). BoNT botulinum toxin, AE adverse event, ROR reporting odds ratios.