Figure 1.
Diagrams Representing Step-by-Step Operating Procedures of the 4D Cell-Culture Insert Array Shape Transformation
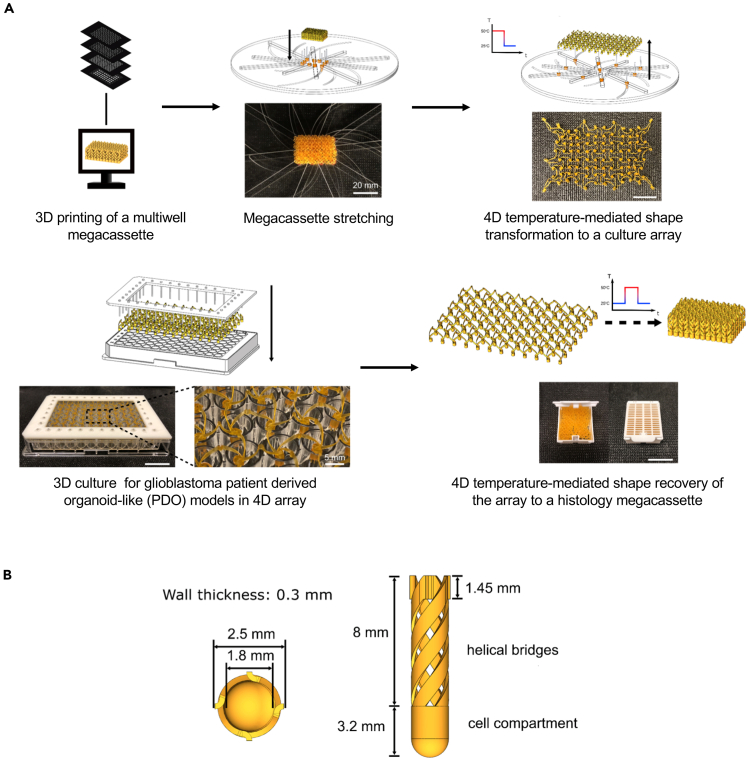
(A) At room temperature (RT), an insert in the cassette configuration was first mounted on a custom-built stretcher. The stretcher has eight rails that can simultaneously move all carriages sitting in rails between the dimension of a cassette and the dimension of a 96-well plate. The insert was stretched to a 96-well plate configuration by simply rotating the top and bottom plates against each other at RT. All helical bridges are unwound during the rotation of the stretcher. After rotation, both the insert and the stretcher were placed in an oven at 50°C for 10 min and then cooled down to RT to program the stretched shape. The insert array was then removed from the stretcher with the temporarily programmed shape. Cell-culture insert arrays were then mounted onto a fixture that has a perfect matching between edge units of the insert and a 96-well plate. The fixture with the insert was then placed on a 96-well plate for cell seeding. Cells, culture media, and treatment compounds were injected into insert wells using micropipettes (see Transparent Methods). After 3D cell culture, the insert was removed from the fixture and heated to 50°C to induce a shape recovery to the cassette configuration while maintaining the registry of the cultured cells. In the cassette configuration, the insert was ready for histological processing to obtain the histology of the entire cell-culture array.
(B) The diagram displays top and side views and the corresponding dimensions of an individual well component of the 4D printed cell-culture insert array. See also Figures S1 and S4 and Video S1 for demonstrations of the thermomechanical properties of the 4D printed cell-culture insert array and Figures S5–S7 for data determining the biocompatibility of these cell-culture arrays.