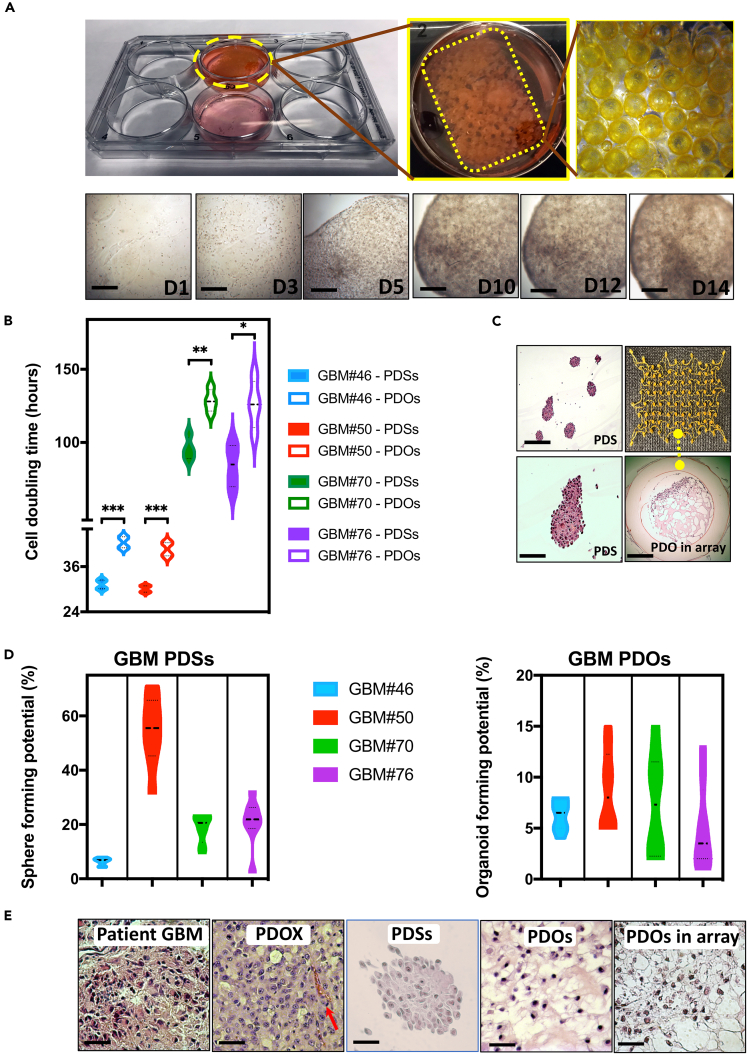
Figure 2.
Utilization of SMP Cell-Culture Insert Arrays for Histological Processing of GBM-PDOs
(A) Diagram displaying the cell-culture array when used in 6-well plates, and bright-field images of 14-day 3D PDO culture. Complexity of the organoid structures increased with time.
(B) Average cell doubling times during the first week of PDS and PDO culture from four patients with GBM.
(C) After 6–14 days of 3D PDS and/or PDO culture alone or in the array, PDSs and PDOs were fixed and stained with H&E. Images demonstrate a group of representative PDSs derived from GBM#50, with the lower image being a 4× magnification of a PDS in the top image. The lower right image demonstrates the phenotypes of PDOs from GBM#46 when cultured in the array.
(D) Sphere- and organoid-forming potentials were assessed using extreme limiting dilution analysis software at http://bioinf.wehi.edu.au/software/elda/. The frequencies based on log-fraction plot of the dilution model, log-active cell fractions, and 95% confidence interval are indicated from four GBM-PDSs and GBM-PDOs.
(E) Histological H&E analysis of original tissue (GBM#46), PDOXs generated from the same patient cells, PDSs, PDOs, and PDOs in the cell-culture insert array collected and fixed after 14 days of culture. Note that the cell density is different in patient and PDOX sections because the cell density depends on the number of engrafted or plated cells. See additional features of these PDSs and PDOs in Figure S2.
Comparison of doubling times and clonogenic potentials between PDSs versus PDOs in (B and D) are represented as mean ± SD of four replicates and were determined by two-way ANOVA with Bonferroni post-hoc test (∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05). Scale bars, 100 μm in (A, C, and E).