Figure 4.
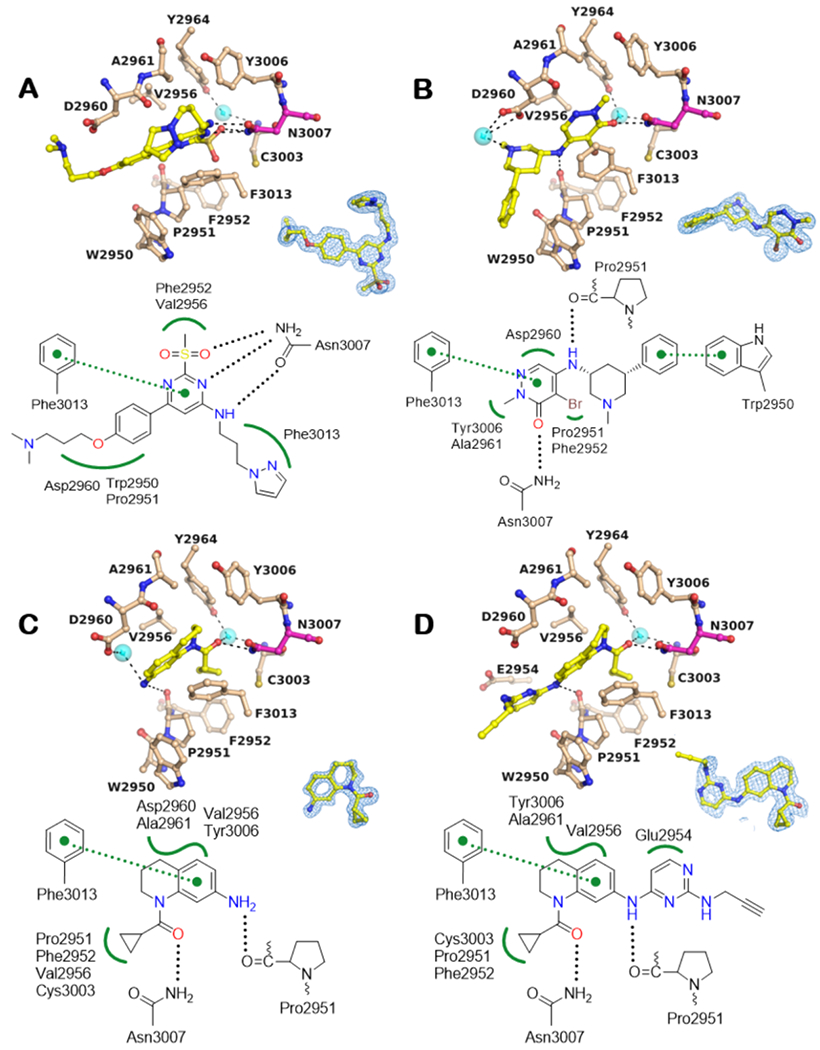
BPTF Bromodomain cocrystal structures. A -D: Cocrystal structures with compounds 2, 3, 4 and 5, respectively.
BPTF residues and small molecules are depicted in beige and yellow, respectively. Potential H-bonding interactions include conserved N3007 (magenta) in the acetylated lysine binding pocket and the main chain carbonyl oxygen of Pro2951 (black dotted lines, 2.2 < d < 3.5 Å). Potential hydrophobic VDW interactions include residues of the WPF shelf (2950-2952) among other residues (green wiggled lines) and Pi-Pi interactions with F3013 green dotted lines with distance cut-off 3.3 < d < 4.0 Å. Cyan spheres show bound water molecules. The blue mesh around the inhibitor shows the corresponding 2Fo-Fc electron density map contoured at 1α. Water mediated H-bonds were excluded from the interaction schematics for clarity.
