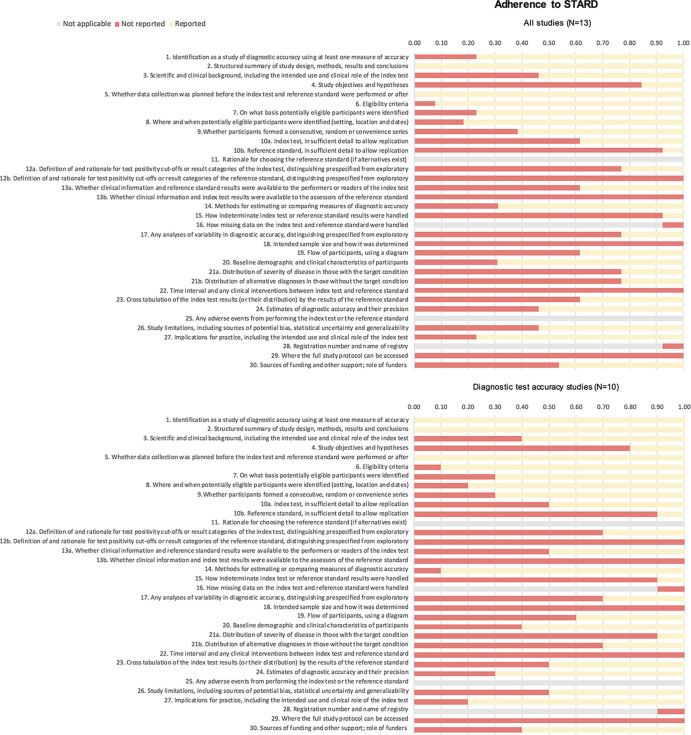
Figure 4:
Adherence to Standards for Reporting of Diagnostic Accuracy Studies (STARD). Presented are the proportions of (non)reported items for each study according to the STARD guidelines; presented for all studies (top) and diagnostic test accuracy studies (bottom). The different STARD items concern the following sections in the reports: title or abstract (1), abstract (1,2), introduction (3), methods (4–18), results (19–25), discussion (26,27), and other (28–30).