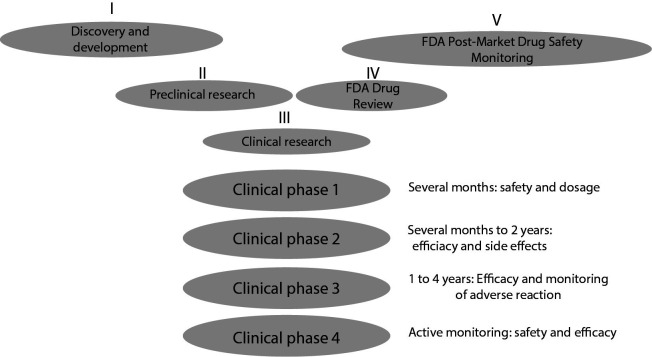
Figure 2.
Phases of drug development (www.fda.gov). Development of drug is finished with preclinical in vitro and in vivo studies. Human drug effects are tested in clinical environment on patients with the condition/disease. Phases are divided into 4 phases: In phase 1 safety and dosage of the drug are determined on few subjects. In phase 2 efficacy and side effects are determined. If passed, drug goes into next phase that lasts from 1 to 4 years where efficacy and adverse reactions are monitored. In 4th phase the drug is ready for the market, safety and efficacy are actively monitored. Food and Drug Administration (FDA) has to review drug documentation and later on monitor drug safety post-market.