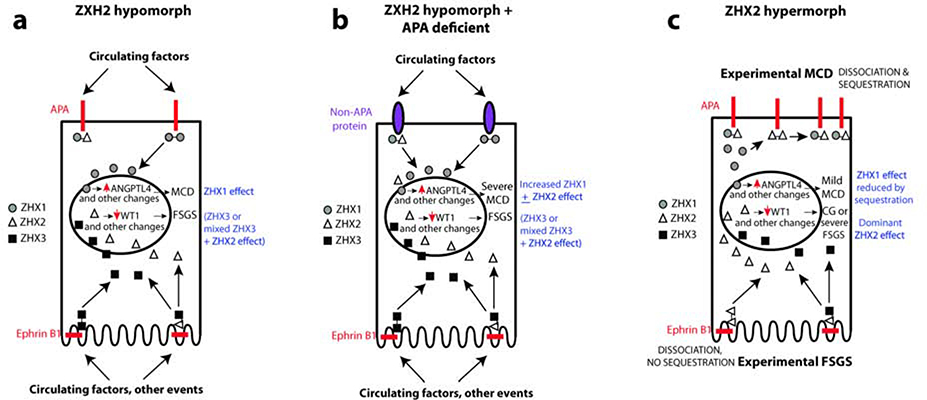
Figure 7: Schematic representation of podocyte ZHX changes in MCD and FSGS.
(a) In a Zhx2 hypomorph state, higher ZHX1:ZHX2 ratio in the podocyte body cell membrane, and ZHX3:ZHX2 ratio in the slit diaphragm alters stability of the ZHX protein complex at the cytoplasmc aspect of APA and EPHRIN B1, respectively. Exposure to disease inducing stimuli or circulating proteins at the podocyte body cell membrane (MCD) or slit diaphragm (FSGS) induces dissociation of ZHX proteins from these transmembrane partners, followed by entry of ZHX1 (MCD) and ZHX3 alone or in combination with ZHX2 (FSGS) into podocyte nuclei. ZHX1 induces Angptl4 upregulation, whereas the ZHX3 + ZHX2 induces FSGS - related changes. (b) In a dual Zhx2 hypomorph and APA deficient state, ZHX proteins in the podocyte body cell membrane bind to alternative putative transmembrane proteins with lower affinity. Following exposure to MCD inducing stimuli, there is greater entry of ZHX1 + ZHX2 into podocyte nuclei, both of which are capable of inducing Angptl4 upregulation, causing more severe MCD. (c) In the experimental Zhx2 hypermorph state, transmembrane protein bound ZHX2 homodimers are increased. During experimental MCD, these homodimers in the podocyte body dissociate and re-associate with ZHX1 cleaved from ZHX1-ZHX2 heterodimers to sequester some ZHX1 in the periphery, resulting in less severe ZHX1 mediated MCD-like changes. In experimental FSGS, ZHX2 homodimers in the slit diaphragm dissociate and migrate into the nucleus to induce changes similar to collapsing glomerulopathy (CG) or severe FSGS in a dose-dependent manner.