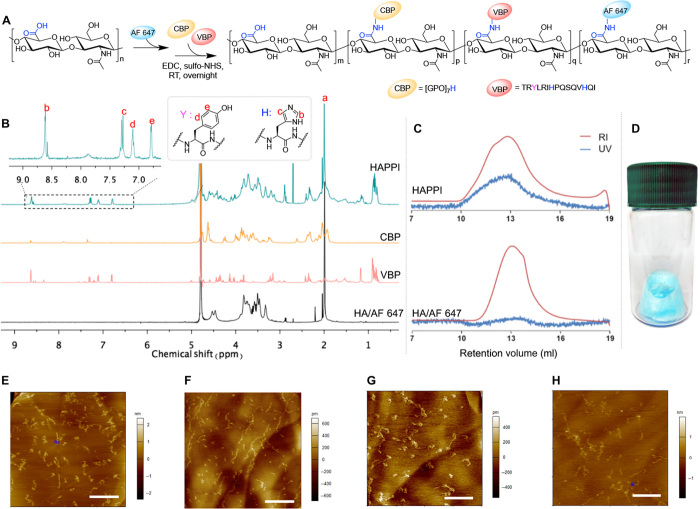
Fig. 1. Synthesis and characterization of AF 647–labeled HA-CBP-VBP conjugates (HAPPI).
(A) Reaction scheme for conjugation of fluorescent dye and peptides on HA backbone using EDC/sulfo-NHS chemistry. (B) 1H nuclear magnetic resonance (NMR) spectrum shows the presence of characteristic peaks b, c, d, and e from the peptides, indicating their successful conjugation to HA. (C) GPC profile of HAPPI shows the appearance of ultraviolet (UV) absorbance at the elution time of conjugates [refractive index (RI) signals], in contrast to the profile of fluorescent HA alone, further validating the chemical conjugation. (D) Image of freeze-dried HAPPI as the bluish material in the vial. Topographic air tapping mode atomic force micrographs for HA (E), HA-VBP (F), HA-CBP (G), and HAPPI (H) (scale bars, 500 nm). Photo credit: (D) Y. Gao, Harvard University.