Abstract
Introduction
Small cell osteosarcoma (SCOS) is a rare subtype of osteosarcoma, with limited studies mainly focusing on histological features. Our study aims to analyze our own patients and those reported in the literature to increase the recognition of this rare disease, to evaluate patient survival and to further determine potential prognostic factors.
Material and methods
Twenty patients with SCOS were treated in our hospital between 2010 and 2019. Their follow-up data were collected retrospectively. A total of 336 literature cases from 58 manuscripts were retrieved by means of a PubMed search with the key word “small cell osteosarcoma”. Data pertaining to treatment and follow-up were extracted. We performed a pooled analysis for the survival of patients and the risk factors for local recurrence (LR), as well as metastatic disease (MD), in a total of 160 patients using the Kaplan-Meier method and Cox regression method.
Results
We reported our experience in diagnosing and treating SCOS. In our cases, elevated alkaline phosphatase (P = 0.013) and lactate dehydrogenase (P = 0.001) significantly impaired overall survival. In the pooled analysis, SCOS was diagnosed at the median age of 17 years and affected both sexes almost equally. The median follow-up duration was 19.5 months. In the pooled analysis cases, the 5-year overall survival rate was 38.6%, and 36.4% of patients survived 10 years. However, an increasing trend was detected, indicating recent improvements in management. The surgical margin status (P = 0.024) and metastases (P = 0.008) significantly impaired overall survival, and the response to chemotherapy was related to disease-free survival (P = 0.012). LR and MD were significantly correlated (P = 0.002) and could be observed after 5 years of follow-up. LR was significantly dependent on response to chemotherapy (P = 0.020). The development of MD seemed to be affected by response to chemotherapy (P = 0.060). Correlations between imaging features and prognosis were not detected.
Conclusions
This study suggested that positive margins, poor response to chemotherapy and MD are negative prognostic factors for SCOS, implied the potential role of laboratory examinations in the survival prediction and supported the need for prolonged or more intensive surveillance in patients with MD or LR. More well-documented literatures are encouraged to allow further confirmations.
Abbreviations: ALP, alkaline phosphatase; CT, computed tomography; DFS, disease-free survival; LDH, lactate dehydrogenase; LR, local recurrence; MD, metastatic disease; MRI, magnetic resonance imaging; OS, overall survival; SCOS, small cell osteosarcoma
Keywords: Neoadjuvant chemotherapy, Metastasis, Recurrence, Small cell osteosarcoma, Surgical margin, Survival
1. Introduction
Small cell osteosarcoma (SCOS), a rare subtype of osteosarcoma, was first described as a distinct clinicopathological entity as a form of “osteosarcoma with small cells simulating Ewing’s sarcoma” by Sim et al [1]. It is generally believed to account for 1.5% of all osteosarcomas, with more than half occurring in the metaphysis of long bones. SCOS affects patients aged 5 to 83 years and is most frequently seen in patients in their second or third decades of life, with a slight predominance in females [2].
SCOS is composed of small cells with scant cytoplasm associated with osteoid production [2] and is histologically difficult to distinguish from other small round cell malignancies, especially Ewing’s sarcoma, malignant lymphoma and mesenchymal chondrosarcoma [3]. Calcification in the intramedullary cavity or an associated extraosseous soft-tissue mass, reflecting the osteoid matrix produced by tumor cells, occurs frequently and is helpful for the differential diagnosis of SCOS from other small cell tumors [4], [5]. Occasionally, nonrepresentative histology sections have demonstrated sheets with no tumor osteoid production, which might lead to misinterpretation as Ewing’s sarcoma; however, uncorrelated radiological features indicating new tumor bone may eliminate that diagnosis [6]. Other radiographic features are not distinctive, such as permeative lytic bone destruction, a soft-tissue mass and periosteal reaction, since they are similar to conventional osteosarcomas [4]. To the best of our knowledge, most of the previous studies on SCOS focused on histological features, and imaging features were not the major consideration [1], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15].
The most important reasons for making the distinction between SCOS and other small cell tumors are therapy and the related prognosis [1]. Exquisitely radiosensitive Ewing’s sarcomas require radiation for local control, but osteosarcomas are almost always insensitive to radiotherapy [15]. As Nakajima et al [11] reported, the 5-year survival rate in their institute was only 28.6% based on an analysis of all of their cases, but patients treated since 1975 might benefit from chemotherapy, which showed a statistically significant advantage in survival. A similar trend might be reflected by results reported recently. The studies reported that the survival rate of SCOS was 41.6% for 5 years in 2004 [16] and 52.6% for 10 years in 2015 [17], implying an improvement in survival. However, limited studies with a survival analysis of SCOS have been documented so far [1], [12], and related factors have seldom been analyzed. Therefore, it is necessary to clarify the factors that might be related to prognosis.
To increase the recognition of this rare disease, we reported our experience in diagnosing and treating SCOS in 20 patients. Moreover, we attempted to evaluate their survival rate and further determine related factors based on a detailed literature review and our own experiences.
2. Materials and methods
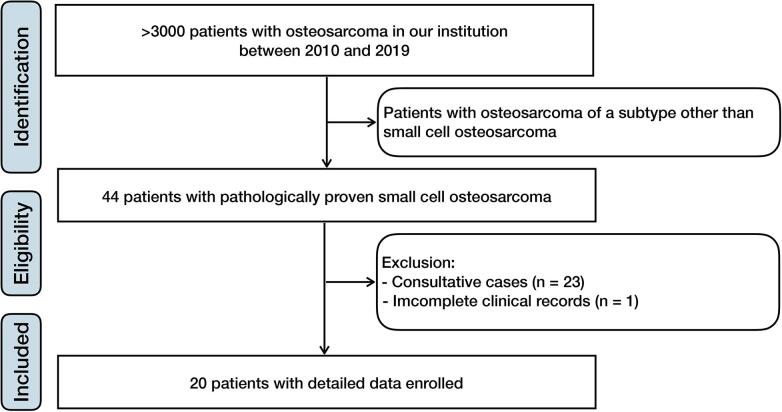
We retrospectively reviewed 20 patients with pathologically proven SCOS who had been treated in our institution between 2010 and 2019 (Fig. 1). The diagnosis was established based on a biopsy and confirmed by surgery if operation was performed. Molecular investigations were performed to approach the diagnosis if necessary. All images were reviewed by two radiologists, and the tumor features were recorded based on expert consensus. The lesion was staged according to the surgical staging system established by Enneking et al [18]. Tumor necrosis was graded based on various axial sections, and patients were divided into good or poor responders [19]. The relevant clinical features and treatment information were retrospectively collected. All patients were followed-up by imaging examinations on an outpatient basis.
Fig. 1.
Flowchart of patient inclusion. Twenty patients with pathologically proven small cell osteosarcoma who had been treated in our institution between 2010 and 2019 were included.
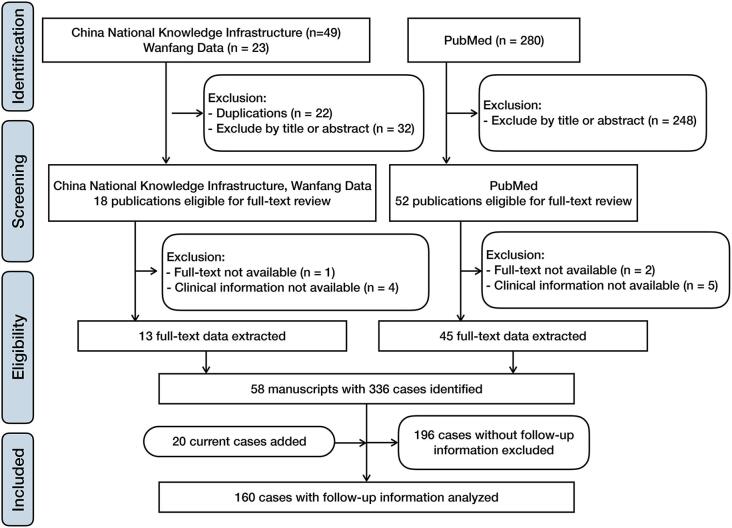
The literature review was performed via the PubMed, China National Knowledge Infrastructure and Wanfang Data databases with “small cell osteosarcoma” as the keyword. Studies in languages other than English, Japanese, German or Chinese and those published before 1979 were excluded. The initial search found 50 studies in Chinese and 280 studies in English after exclusion of duplicates. The titles and abstracts were screened to determine eligibility, full texts were reviewed, and study data were extracted. The reference lists of the included studies were screened for additional potentially eligible articles. In total, 58 manuscripts with 336 cases were identified [6], [7], [8], [9], [11], [12], [13], [14], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67]. Follow-up information was available for 140 patients (Fig. 2).
Fig. 2.
Flowchart of pooled analysis. A total of 336 cases of small cell osteosarcoma extracted from 58 manuscripts were identified. A total of 140 patients with follow-up information and 20 patients in our institution were pooled and analyzed.
All analyses were performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). The data of current cases were merged with the literature data. All cases with missing data were excluded from the analysis. Continuous variables were reported as the median and range, and categorical variables were reported as percentages and frequency distributions; the chi-square test was used for categorical variables, and Fisher’s exact test was used for those groups with small sample sizes. Survival analysis was estimated using the Kaplan-Meier method based on available patient-level data. Significance analysis was performed by the log-rank test or chi-square test using a 95% confidence interval. Multivariate analysis by the Cox regression method was used for prognostic factors that had statistical significance in prior analyses. All of the statistical tests were 2-sided, and a P value of < 0.05 was considered statistically significant.
The ethics committee of our institution approved this study, and it conformed to the provisions of the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective nature of the study using deidentified patient data.
3. Results
3.1. Current cases
The detailed clinical characteristics of patients are summarized in Table 1. Sixteen males and four females were enrolled in our study with a median age of 23 years, ranging from 8 to 69 years. The most frequently involved site was the femur. In fifteen cases involving long bones, nine cases involved the metaphysis. Patients presented with pain, masses and swelling. Notably, there was one patient with a family history of cancer and another patient with a history of radiation therapy.
Table 1.
Clinical details of 20 patients with SCOS.
| Case | Sex | Age (year) | Presentation (duration) | Location (site in long bone) | Maxium size (cm) | Enneking Staging | ALP | LDH | Treatment | Chemotherapy Protocol | Response to chemotherapy | Margin of resection | Metastasis (interval) | Recurrence (interval) | Follow-up (duration) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 21 | Pain (2 m) | R prox fum (meta) | 7.8 | IIB | nor | nor | NC + WRA + C | DOX | good | wide | n | n | ANED (126 m) |
| 2 | F | 29 | Pain (2 m) | L dist fum (meta) | 2.0 | IIB | nor | nor | NC + WRA + C | THP | good | wide | n | n | ANED (123 m) |
| 3 | F | 39 | Pain (3 m) | L mid tibia (dia) | 3.9 | IIB | nor | nor | NC + EPR + C | DOX | good | radical | lung (95 m) | n | AWD (99 m) |
| 4 | M | 48 | Pain (24 m) | L prox tibia (mata) | 9.9 | IIB | abn | abn | NC + amputation | DOX | poor | wide | n | n | ANED (7 m) |
| 5 | M | 14 | Injury (2d) | R diat fum (meta) | 8.6 | IIB | abn | abn | NC + WRA + C + amputation | THP | poor | wide + intralesional | lung (15 m) | 2 m; 8 m | DOD (16 m) |
| 6 | M | 60 | Swelling (5 m) | R diatal tibia (meta) | 5.2 | IIB | abn | nor | NC + WRA + C + amputation | THP | poor | wide | multiple (18 m) | 10 m | DOD (37 m) |
| 7 | M | 25 | Swelling (5 m) | R prox hum (meta) | 9.4 | IIB | nor | abn | NC + WRA + C | DOX | good | wide | n | n | ANED (85 m) |
| 8 | M | 31 | Mass (4 m) | R pelvis | 7.5 | IIB | abn | nor | NC + Te + C + R | DOX | poor | marginal | lung (3 m) | 3 m | DOD (6 m) |
| 9 | M | 49 | Swelling (1 m) | L dist fum (meta) | 6.4 | III | abn | nor | NC + WRA + C | THP | poor | wide | n | n | ANED (70 m) |
| 10 | F | 15 | Pain (6 m) | R prox tibia (dia) | 4.4 | III | nor | nor | NC + WRA + C | DOX | good | wide | n | n | ANED (52 m) |
| 11 | M | 19 | Mass (1 m) | R pelvis | 8.9 | III | abn | abn | NC + TCAE + EPR + C | VAC/IE, DOX | good | marginal | bone (4 m) | n | DOD (40 m) |
| 12 | M | 17 | Pain (8 m) | L pelvis | 18.3 | III | abn | abn | NC + amputation | DOX | poor | marginal | n | n | DOD (4 m) |
| 13 | F | 20 | Mass (4 m) | L mid-dist fum (dia) | 5.8 | IIB | nor | nor | NC + resection + C | DOX | poor | radical | n | n | ANED (53 m) |
| 14† | M | 18 | Pain (1 m) | L dist fum (dia) | 9.5 | IIB | abn | nor | NC + WRA + C + amputation | THP | poor | wide + marginal | lung (2 m) | 11 m | DOD(12 m) |
| 15 | M | 25 | Pain (4 m) | R pelvis | 14.1 | III | abn | abn | NC | DOX | poor | n | lung (1 m) | n | DOD(7 m) |
| 16‡ | M | 40 | Pain (9 m) | L dist fum (dia) | 18.8 | III | abn | abn | NC + hemipelvectomy + C | THP | poor | intralesional | lung (7 m) | n | DOD (6 m) |
| 17 | M | 8 | Pain (2 m) | R diat fum (dia) | 4.2 | IIB | abn | abn | NC + resection + C | DOX | poor | radical | lung (9 m) | 11 m | DOD (17 m) |
| 18 | M | 19 | Pain (3 m) | R prox tibia (meta) | 8.5 | IIB | nor | nor | NC + resection + C | DOX | poor | radical | lung (1 m) | 15 m | DOD (21 m) |
| 19 | M | 14 | Pain (1 m) | R prox fum (meta) | 11.2 | IIB | nor | nor | NC + WRA + C | DOX | good | wide | n | n | AWD (21 m) |
| 20 | M | 69 | Pain (12 m) | L pelvis | 27.2 | III | abn | abn | Te | n | n | intralesional | n | n | DOD (7 m) |
Note: †family history of cancer; ‡previous history of sarcoma treated by radiation.
Abbreviations: abn, abnormal; ANED, alive with no evidence of disease; AWD, alive with disease; C, adjuvant chemothrapy; dia, diaphysis; dist, distal; DOD, died of disease; EPR, limb-salvage surgery with endoprosthetic replacement of resected bone; fum, fumer; hum, humerus; L, left; meta, metaphysis; mid, middle; m, months; n, none; NC, neo-adjuvant chemotherapy; nor, norMEmal; prox, proximal; r, radiotherapy; R, right; SCOS, small cell osteosarcoma; TCAE, transcutaneous arterial embolization; Te, tumor exclusion; WRA, wide resection and arthroplasty; y, years; DOX, doxorubicin-based osteosarcoma directed chemotherapy protocol; THP, pirarubicin-based osteosarcoma directed chemotherapy protocol; VAC/IE, an Ewing sarcoma directed chemotherapy protocol with vincristine, doxorubicin, cyclophosphamide, dactinomycin, ifosfamide and etoposide.
For laboratory examinations, 12 out of 20 patients had an elevated alkaline phosphatase (ALP) level; 9 out of 20 patients had an elevated lactate dehydrogenase (LDH) level before treatment. Eight patients demonstrated both abnormal ALP and LDH levels.
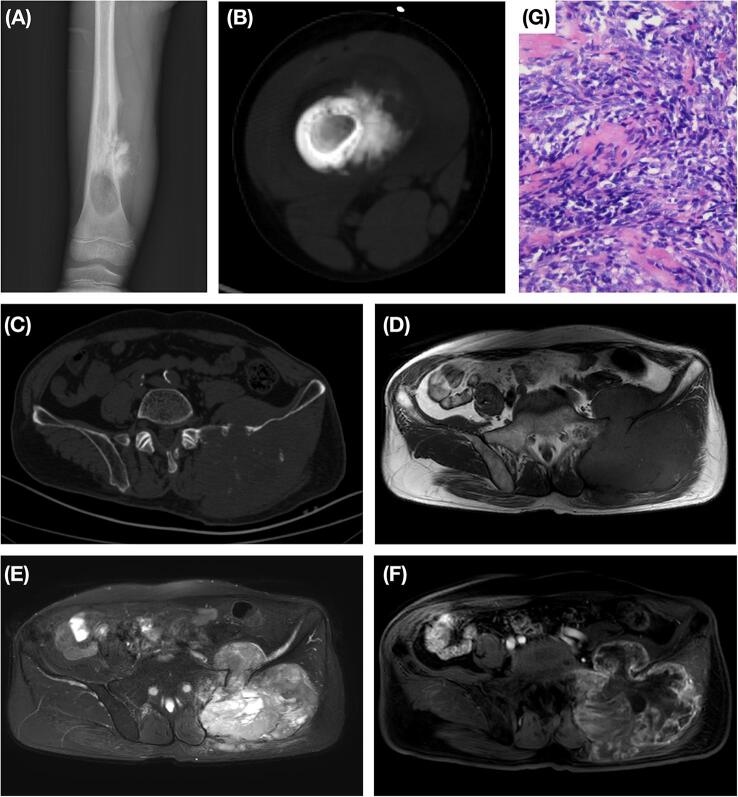
On radiographs or CT scans, all tumors showed a slight lytic component and six presented with an associated osteogenic component (Fig. 3 A-B). Matrix mineralization was found in sixteen of the tumors, ten of which showed mineralization in the associated soft-tissue mass to support the diagnosis of osteosarcoma (Fig. 3 C). Periosteal reaction was detected in thirteen of our cases, and pathologic fracture was found in nine cases. On MRI, tumors were typically isointense to hypointense homogeneous lesions on T1-weighted imaging and hyperintense heterogeneous lesions on T2-weighted imaging compared to muscle. After contrast administration, most tumors exhibited an intermediate to notable heterogeneous enhancement pattern. A soft-tissue mass was detected in fifteen cases, and slight soft tissue edema was found in eight cases (Fig. 3 D-F).
Fig. 3.
Radiological and pathological findings of SCOS. A-B, Case 17, an 8-year-old boy. Radiograph and CT scan showed a lytic lesion of the right distal femur with an associated soft-tissue mass with an osteogenic component. Periosteal reaction was detected. C-G, Case 20, a 69-year-old male. CT scan demonstrated a soft-tissue mass with mineralization associated with the left ilium (C). The tumor was found to be homogeneously isointense on T1-weighted imaging and heterogeneously hyperintense on T2-weighted imaging when compared to muscle (D, E). A significant heterogeneous peripheral enhancement pattern was detected after contrast administration (F). High-magnification images (hematoxylin-eosin staining, 400×) showed short spindle-shaped tumor cells with osteoid production (G).
Pathological diagnoses were confirmed in nineteen cases after surgery and in one case with biopsy. Grossly, the tumors showed a gray-white appearance with invasive growth patterns. Histologically, round, oval or short spindle tumor cells were distributed in a patchy or diffuse manner with unclear cell boundaries. The presence of osteoid production directly by the small tumor cells was detected in all cases and is the most distinctive feature conferring the diagnosis of osteosarcoma (Fig. 3 G). The osteoid deposition was scantly distributed in a lace-like pattern, and broad sheets of osteoid were not found. Immunohistochemically, STAB2 (19/19) and vimentin (16/16) were positive in all cases, and two-thirds of all cases expressed CD99 (12/18); S-100, CK, CD45, Syn and SMA immunoreactions were seldom detected. In two cases, the EWSR1 gene test was performed, and no rearrangement was detected.
All patients were treated under the direction of a multidisciplinary team. Nineteen patients received neoadjuvant chemotherapy before surgery, and one of the patients was further treated with transcutaneous arterial embolization. Eighteen patients were treated with surgical resection after chemotherapy, one patient came to us only for palliative care, while one patient refused surgery due to initial lung metastasis and poor general condition. Sixteen patients continued to receive adjuvant chemotherapy after surgical treatment, and one patient also underwent radiotherapy. Three patients underwent reoperation.
In nineteen patients treated with chemotherapy, eighteen of them received chemotherapy with an optimized osteosarcoma-directed doxorubicin- or pirarubicin-based protocol [68], [69]; one of them received an Ewing’s sarcoma-directed chemotherapy [70], due to misdiagnosis. After surgery, the pathologists corrected their diagnosis to SCOS. Then the patient received an adjuvant chemotherapy with doxorubicin -based protocol with a delay of 2 months.
Follow-up information was available for all patients. The median duration of follow-up was 21 months, ranging from 4 to 126 months. Local recurrence (LR) was detected in six patients, and one of them experienced LR twice; metastatic disease (MD) was found in ten patients. The predominant site of MD was the lung.
The median disease-free survival (DFS) was 18 months, and the median overall survival (OS) was 21 months. The 5-year survival rate was 41.7% in our case series. Abnormal ALP was found to be related to MD (p = 0.010) and negatively influenced DFS (7.0 vs not evaluable, p = 0.005). Abnormal LDH and ALP both had negative impacts on OS (7 vs 21 months, p = 0.013; 7 vs 21 months, p = 0.001).
3.2. Pooled analysis of data
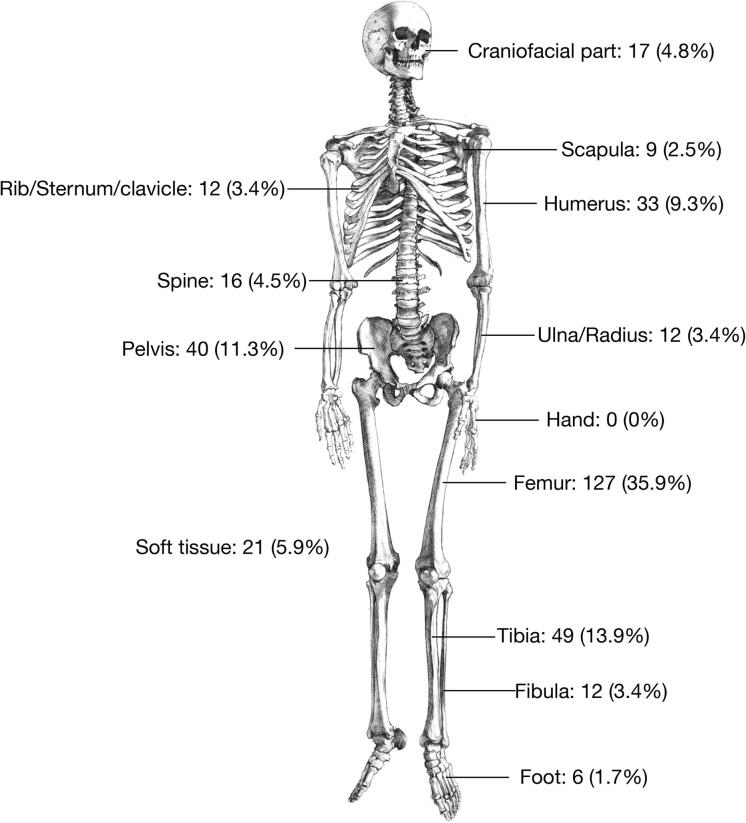
A total of 336 cases were identified, including 154 males, 161 females and 21 patients of unknown gender (Table 2). Taking our own 20 cases into consideration, the gender ratio was 1.03:1 (170 male and 165 female). The median age at the time of diagnosis was 17 years, ranging from 5 to 83 years. The most affected site was the femur (35.9%), followed by the tibia and pelvis (Fig. 4). Tumor size was available for 29 patients and ranged from 2 to 27.2 cm, with a median of 7.5 cm. Imaging features were available for 46 patients. A total of 36.7% (11/30) of patients had pathological fractures, 73.8% (31/42) had periosteal reactions, and 73.9% (34/46) showed mineralization.
Table 2.
Included studies.
| Author [Reference] | Year | Patients (n) | Gender (M/F) | Age (year) | Outcome |
Follow-up (months) | Local recurrence (LR) |
Metastatic disease (MD) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANED | AWD | DOD | LR (n) | Time to LR (months) | MD (n) | Time to MD (months) | ||||||
| Sim et al. [1] | 1979 | 24 | 12/12 | 9–83; average 28 | 4 | 2 | 18 | 1–75; median 25.5 | 0 | N/A | 3 | 0–120; median 19 |
| Martin et al. [7] | 1982 | 6 | 1/5 | 6–31; median 15 | 4 | 0 | 2 | 0–120; median 22 | 0 | N/A | 1 | 13 |
| Giangaspero et al. [20] | 1984 | 1 | 0/1 | 8 | 1 | 0 | 0 | 24 | 0 | N/A | 1 | N/A |
| Roessner et al. [21] | 1985 | 1 | 0/1 | 29 | 0 | 0 | 1 | 204 | 1 | 192 | 1 | 168 |
| Ediken et al. [6] | 1987 | 13 | 5/8 | 6–28; median 15 | 5 | 3 | 5 | 2–84; median 20.5 | 0 | N/A | 4 | 6–24 |
| Sanjay et al. [22] | 1987 | 1 | 1/0 | 10 | 0 | 0 | 1 | 7 | 0 | N/A | 1 | 2 |
| Stea et al. [15] | 1988 | 8 | 2/6 | 6–31; median 17 | 3 | 1 | 4 | 10–216; median 57 | 1 | 60 | 5 | 9–120; median 22 |
| Ayala et al. [9] | 1989 | 27 | 12/15 | 6–28; median 14 | 12 | 1 | 14 | 1–115; median 20 | 0 | N/A | 17 | N/A |
| Bertoni et al. [8] | 1989 | 11 | 4/7 | 6–20; median 13 | 1 | 0 | 8 | 3–18; median 6 | 1 | 3 | 8 | 0–14; median 3 |
| Nguera et al. [23] | 1990 | 1 | 1/0 | 20 | 0 | 0 | 1 | 10 | 0 | N/A | 1 | 10 |
| Dickersin et al. [24] | 1991 | 4 | 3/1 | 17–39; median 25 | 0 | 2 | 2 | 4–78; median 12 | 0 | N/A | 1 | 72 |
| Huang et al. [25] | 1991 | 1 | 1/0 | 34 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Kyriakos et al. [26] | 1992 | 1 | 1/0 | 15 | 1 | 0 | 0 | 57 | 0 | N/A | 0 | N/A |
| Devaney et al. [27] | 1993 | 16 | N/A | 16–28; average 19 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Stracca-Pansa et al. [28] | 1994 | 4 | 3/1 | 3–11; mean 7.5 | N/A | 1 | N/A | N/A | N/A | N/A | N/A | N/A |
| Park et al. [29] | 1995 | 1 | 0/1 | 8 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Robinson et al. [30] | 1995 | 1 | 0/1 | 30 | 1 | 0 | 0 | 9 | 0 | N/A | 0 | N/A |
| Nakajima et al. [11] | 1997 | 72 | 39/33 | 5–71; median 20 | 4 | 0 | 14 | 3–199 | 0 | N/A | 1 | 53 |
| Sipos et al. [31] | 1997 | 1 | 0/1 | 16 | 1 | 0 | 0 | 48 | 0 | N/A | 0 | N/A |
| Ye [32] | 1997 | 1 | 1/0 | 40 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Tian et al. [33] | 1998 | 1 | 1/0 | 14 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Lintz et al. [34] | 1999 | 1 | 1/0 | 32 | 0 | 0 | 1 | 36 | 0 | N/A | 1 | 0 |
| Mulligan et al. [35] | 1999 | 1 | 1/0 | 63 | 0 | 0 | 1 | 26 | 0 | N/A | 1 | 17 |
| Park et al. [36] | 1999 | 1 | 0/1 | 37 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Todesco et al. [37] | 2000 | 1 | 1/0 | 12 | 1 | 0 | 0 | 33 | 0 | N/A | 1 | 15 |
| Nakamura et al. [38] | 2001 | 1 | 1/0 | 36 | 1 | 0 | 0 | 95 | 0 | N/A | 1 | 48 |
| Cai et al. [39] | 2002 | 1 | 0/1 | 23 | 0 | 0 | 1 | 14 | 0 | N/A | 1 | N/A |
| Zhang et al. [40] | 2003 | 1 | 1/0 | 16 | 1 | 0 | 0 | 24 | 0 | N/A | 0 | 0 |
| Nishio et al. [41] | 2006 | 1 | 1/0 | 9 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Weng et al. [42] | 2006 | 1 | 1/0 | 18 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Fang et al. [43] | 2009 | 1 | 1/0 | 24 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Li et al. [44] | 2009 | 1 | 0/1 | 32 | 1 | 0 | 0 | 12 | 0 | N/A | 0 | 0 |
| Kallel et al. [45] | 2009 | 1 | 1/0 | 14 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Yang et al. [46] | 2009 | 1 | 1/0 | 31 | 1 | 0 | 0 | 24 | 0 | N/A | 0 | N/A |
| Bishop et al. [13] | 2010 | 5 | N/A | 11–37 | 1 | 1 | 2 | up to 48 | 1 | N/A | 1 | N/A |
| Machado et al. [14] | 2010 | 10 | 4/6 | 5–48; median 28 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| PosthumaDeBoer et al.[47] | 2010 | 1 | 1/0 | 30 | 1 | 0 | 0 | 6 | 0 | N/A | 1 | N/A |
| Qi et al. [48] | 2010 | 1 | 1/0 | 31 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Debelenko et al. [49] | 2011 | 1 | 0/1 | 12 | 0 | 1 | 0 | 36 | 0 | N/A | 1 | 0 |
| Uma et al. [50] | 2011 | 1 | 0/1 | 28 | N/A | 1 | N/A | N/A | N/A | N/A | N/A | N/A |
| Ali et al. [51] | 2012 | 1 | 0/1 | 36 | 0 | 1 | 0 | 5 | 0 | N/A | 1 | 0 |
| Findik et al. [52] | 2012 | 1 | 0/1 | 28 | 1 | 0 | 0 | 17 | 0 | N/A | 0 | N/A |
| Futani et al. [53] | 2012 | 1 | 1/0 | 11 | 1 | 0 | 0 | 48 | 0 | N/A | 0 | N/A |
| Huang et al. [54] | 2012 | 14 | 7/7 | 12–73; median 29 | 1 | 1 | 1 | 12–15; median 15 | 1 | 12 | 0 | 0 |
| Jambhekar et al. [55] | 2012 | 1 | 0/1 | 17 | 0 | 1 | 0 | 9 | 0 | N/A | 1 | 5 |
| Pugi et al. [56] | 2012 | 1 | 1/0 | 11 | 1 | 0 | 0 | 12 | 0 | N/A | 0 | N/A |
| Kang et al. [57] | 2013 | 1 | 1/0 | 16 | 0 | 0 | 1 | 42 | 1 | 10 | 1 | 10 |
| Wan et al. [58] | 2013 | 12 | 6/6 | 11–59; median 27 | 3 | 3 | 0 | 4–55; median 15.5 | 1 | 4 | 0 | 0 |
| Sood et al. [59] | 2014 | 1 | 0/1 | 60 | 1 | 0 | 0 | 6 | 0 | N/A | 0 | N/A |
| Zhang et al. [60] | 2014 | 1 | 0/1 | 40 | 1 | 0 | 0 | 36 | 0 | N/A | 0 | N/A |
| Agarwal et al. [61] | 2015 | 1 | 0/1 | 16 | 0 | 0 | 1 | 6 | 0 | N/A | 0 | 0 |
| Handa et al. [62] | 2015 | 1 | 1/0 | 10 | 1 | 0 | 0 | 6 | 0 | N/A | 0 | N/A |
| Righi et al. [12] | 2015 | 36 | 17 | 7–72 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Chen et al. [63] | 2017 | 1 | 0/1 | 5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Jeong et al. [64] | 2017 | 1 | 1/0 | 31 | 1 | 0 | 0 | 73 | 0 | N/A | 0 | N/A |
| Wang et al. [65] | 2017 | 32 | 16/16 | 13–76; median 30 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| AbdullGaffar [66] | 2018 | 1 | 0/1 | 32 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Syriac et al. [67] | 2019 | 1 | 0/1 | 36 | 0 | 1 | 0 | 96 | 0 | N/A | 1 | 96 |
| Current cases | N/A | 20 | 16/4 | 8–60; median 27 | 7 | 2 | 11 | 4–126; median 21 | 6 | 2–15; median 10.5 | 10 | 1–95; median 5.5 |
| Total | N/A | 356 | 170/165 | 5–83; median 17 | 62 | 22 | 89 | 1–216; median 20 | 13 | 2–192; median 10.5 | 66 | 0–168; median 10 |
Abbreviations: AWD, alive with disease; ANED, alive with no evidence of disease; DOD, died of disease; F, female; LR, local recurrence; M, male; MD, metastatic disease; N/A, not available.
Fig. 4.
Location distribution of pooled analysis cases.
Outcome data could be obtained for 173 cases (51.5%) (Table 2). Sixty-two patients (35.8%) were alive with no evidence of disease, 22 patients (12.7%) were alive with LR or MD, and 89 patients (51.4%) died due to the disease. Thirteen patients experienced LR, and MD was detected in 66 cases. The primary site for MD was the lung.
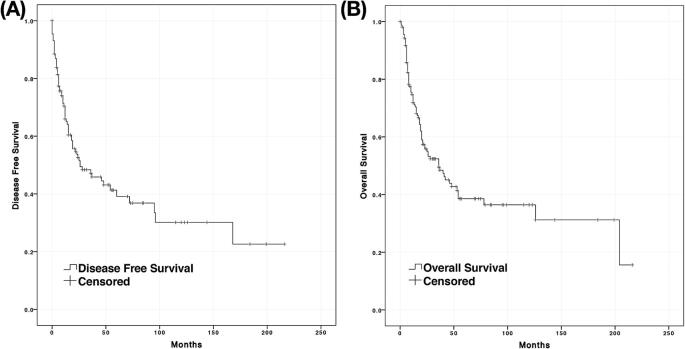
However, detailed follow-up data were only available for 160 cases (47.6%) and further pooled for analysis. The median follow-up duration was 20 months, ranging from 1 to 216 months. The median DFS and OS were 26 and 36 months, respectively (Fig. 5). In total, 38.6% of cases survived 5 years, and only 36.4% of patients survived 10 years. The 5-year survival rate has been on the rise in recent years. Only 14.3% of patients survived for 5 years before 1980, while from 1980 to 2000, the percentage was 40.6%, and after 2000, it was 50.0%.
Fig. 5.
DFS and OS of the pooled analysis cases. A, Disease-free survival. B, Overall survival.
Treatment modalities could be evaluated in all these cases. A total of 126 patients (78.8%) underwent surgery. R0 resection was achieved in 82 cases (65.1%), R1/2 resection was achieved in 32 cases (25.4%), and the margins remained unknown in 12 cases (9.5%). Of the 33 patients who refused surgery, ten were treated with both chemotherapy and radiotherapy, ten with only chemotherapy, nine with only radiotherapy and four refused any treatment. Neoadjuvant chemotherapy was administered in 52 patients (32.5%); the tumor necrosis percentage was available in 31 patients. Thirteen patients were classified as good responders, and 18 patients were classified as poor responders.
In the group of pooled analysis cases, twelve patients (7.5%) experienced LR in the median 10.5 months, ranging from 2 to 192 months, after resection of the tumor. The correlation between the surgical margin and LR could not be detected (p = 0.288), while response to chemotherapy was related to LR (p = 0.020). LR showed no significant influence on OS (p = 0.138).
In the pooled analysis cases, 62 patients (38.8%) had MD. Six of the MD cases were discovered at the time of diagnosis; 36 patients developed metastases during the median follow-up time of 10 months, ranging from 1 to 168 months; and the time until detection of 20 patients was unknown. A significant correlation was found between MD and LR (p = 0.002). However, the correlation between the surgical margin (p = 0.180) and MD could not be detected but seemed to be affected by response to chemotherapy and MD (p = 0.060). MD negatively influenced OS (18 months vs not evaluable, p < 0.001).
With regard to DFS, response to chemotherapy was detected to be significant in univariate analysis. With regard to OS, surgical margin, response to chemotherapy and MD were all suggested to be significant in univariate analysis with pooled data. However, in multivariate analysis, only surgical margin and MD maintained statistical significance (Table 3).
Table 3.
Cox proportional-hazards regression for OS and DFS.
| Survival | Variables | Univariate analysis Hazard ratio (95% confidence interval) | P-value | Multivariate analysis Hazard ratio (95% confidence interval) | P-value |
|---|---|---|---|---|---|
| DFS | Response to chemotherapy: Good vs Poor | 0.140 (0.030–0.646) | 0.012 | – | – |
| OS | Surgical margin: R0 vs R1/2 | 0.512 (0.298–0.881) | 0.016 | 0.153 (0.039–0.607) | 0.024 |
| Response to chemotherapy: Good vs Poor | 0.204 (0.057–0.733) | 0.015 | – | 0.282 | |
| MD: Yes vs No | 3.045 (1.897–4.887) | < 0.001 | 11.155 (1.376–90.430) | 0.008 |
Abbreviations: DFS, disease free survival; OS, overall survival.
4. Discussion
4.1. Diagnostic consideration
SCOS has been defined as a small, round cell mesenchymal malignancy whose characteristic trait is osteoid production directly by tumor cells [1], [12], [14]. The age and skeletal distribution of SCOS were similar to those of conventional osteosarcoma [11]. In our study, this disease affected the patients most in the second decade of life and arose most frequently in the femur, but there was no obvious gender predominance detected.
Imaging features of SCOS, including lytic bone destruction, soft-tissue masses and periosteal reaction, were as frequently seen as in conventional osteosarcoma [4]. Mineralization in the intramedullary cavity or in an associated soft-tissue mass was a helpful diagnostic clue implying that the lesion was producing osteoid matrix [4]. However, this feature was not consistently typical for SCOS because there might be little mineralized matrix produced [5]. In our study, most included cases showed periosteal reactions, and more than one-third of patients presented with pathological fractures. Mineralization, a useful feature for distinguishing between SCOS and Ewing’s sarcoma [4], was detected in most cases.
The diagnosis of SCOS is difficult, particularly in cases with minimal, doubtful or absent osteoid production [5], [12], [14]. All of our cases were diagnosed after the detection of osteoid production by small tumor cells, according to the original definition established by Sim et al [1]. In our study, pink-staining matrix material produce by small round tumor cells, which was considered to be osteoid, were detected and confirmed by experienced pathologists in all of our cases. We retrospectively performed the STAB2 immunostaining to further confirm our findings, except for one case whose sample was not available. All stained cases were positive with SATB2 expression, which is believed to provide one of the key diagnostic clues to distinguish small cell osteosarcomas from other small round cell malignancies of bone [12].
Nonetheless, in the absence of a specific immunoprofile or a known common molecular denominator, differential diagnosis of SCOS has been challenging. The role of CD99 was inconclusive. Although Righi et al [12] found that CD99 expression was consistently negative in their cases, our findings and other studies [13], [14] demonstrated that CD99 could be positive in some SCOS patients and thus should not be used to distinguish SCOS from Ewing’s sarcoma or primitive neuroectodermal tumors [3]. However, other immunoprofiles are useful in distinguishing malignant lymphoma, metastatic small cell carcinoma and other small cell malignancies [3].
The presence of particular molecular signatures led to several distinctive diseases [3]; on the other hand, no reproducible molecular genetic abnormalities were detected in SCOS, but a minority of patients shared either EWSR1 or BCOR rearrangements [3], [12], [14]. Only two cases in our study used EWSR1 gene tests, and no specific genetic abnormalities were detected. Further molecular exploration such as RNA-seq technique may detect transcripts associated with Ewing’s sarcoma, mesenchymal chondrosarcoma, and other small cell tumors.
To date, osteoid production by tumor cells, STAB2 expression and the absence of EWSR1 gene rearrangement are believed to enable the recognition of SCOS in the appropriate clinical settings. SCOS-associated particular molecular signatures has not been detected so far; however, molecular exploration may help the pathologist to exclude familiar small cell tumors.
4.2. Treatment and prognosis
The most important reason to distinguish SCOS from other small cell tumors is the diversity of therapy. SCOS does not appear to be radiosensitive, and radical surgery and chemotherapy could offer the best chance of a cure [1]. Similar to in conventional osteosarcoma, this inference is currently widely accepted but only supported by limited studies focusing on SCOS [1], [6], [11]. Our review of the literature implied that replacement of radiotherapy with chemotherapy resulted in an increasing trend for the survival rate of SCOS (Fig. 6) with a 5-year survival rate over 40%, similar to the results of a database analysis in 2004 [16]. Moreover, the 10-year survival rate of 52.6% calculated in 2015 [17] might further reflect improvements in the treatment in recent years.
Fig. 6.
Therapy options for SCOS. Surgery was performed in more than two-thirds of patients with small cell osteosarcoma. In manuscripts published before 1980, radiotherapy was used as a main adjuvant treatment for those patients; however, radiotherapy was gradually replaced by chemotherapy and used only in 14% of patients in manuscripts published after 2000. Surgery and chemotherapy are currently the main treatments for small cell osteosarcoma.
We further determined prognosis-related factors, and therapy-related factors were the first concern. As a previous study discovered, response to chemotherapy and a surgical margin had independent prognostic value in osteosarcoma [71]. In the pooled analysis, a negative surgical margin was confirmed to be a beneficial factor for OS. However, no relation between a surgical margin and LR was detected; this outcome might be explained by limited LR cases and incomplete records of margin status. The response to chemotherapy was found to be related to LR and had a positive influence on DFS and OS in univariate analysis. However, due to the respective nature of this study, only 31 cases of tumor necrosis were analyzed; therefore, larger studies are needed to verify this conclusion. Furthermore, some patients might not have been appropriately diagnosed at the time they receive treatment, and thus might have received Ewing’s sarcoma-directed chemotherapy rather than osteosarcoma-directed chemotherapy. Several various osteosarcoma-directed protocols used in different studies, as another probable confounding factor, could bias the conclusion.
Metastasis at present was considered a negative predictor for the prognosis of conventional osteosarcoma [71]; however, because only six cases with primary metastases and more than one-third of cases with undetermined metastatic time were recorded, the relationship between primary metastases and OS was not examined. In ten cases, both MD and LR occurred, indicating a significant correlation between MD and LR. Five cases of MD were detected after 5 years, and two cases with LR events were found at 60 and 192 months during follow-up, supporting the need for prolonged or more intensive surveillance in patients with MD or LR. Only MD had a negative influence on OS and maintained statistical significance in multivariate analysis, as indicated in a previous study [12].
The poor prognostic factors detected in SCOS, including positive margins, poor response to chemotherapy and occurrence of MD, seemed very overlapping with conventional high-grade osteosarcoma. On one hand, although SCOS has a distinct histological appearance from conventional osteosarcoma, this subtype of osteosarcoma may hold a similar biological behavior to conventional ones. In our study, we also found that the demographic characteristics, skeletal distribution of SCOS were closed to those of conventional osteosarcoma. These findings indicated that SCOS is a subtype of osteosarcoma, different from other small cell tumors. On the other hand, the poor prognostic factors detected in our study were commonly accepted as those for almost all tumors. Therefore, it is acceptable that those are overlapping with conventional osteosarcoma.
Two additional potential negative prognostic factors for SCOS were ALP and LDH. In our own cases, abnormal levels of ALP and LDH were found for the first time to be related to MD and negatively influence the prognosis of SCOS, in accordance with previous findings in patients with conventional osteosarcoma [72], [73]. Meta-analyses demonstrated that ALP and LDH were related with poor prognosis in osteosarcoma patients, including event-free survival and overall survival [74], [75]. Many researchers thought higher serum ALP and LDH level meant heavier osteosarcoma burden, which implied worse prognosis [74], [75]. However, a pooled analysis was unavailable since laboratory examinations were not the main consideration in case reports and series of SCOS. The potential role of laboratory examinations in SCOS prognosis prediction needs more well-documented studies to allow further research.
We analyzed the role of imaging features in the prognosis of SCOS. Although a recent study observed the occurrence of pathological fracture to correlate with inferior OS in adult patients [76], the predictive value of its presentation and other imaging features in the prognosis of SCOS could not be detected.
4.3. Limitations and future directions
There are certain limitations in our study. As is the case with most of the literature on this subject, only a small number of cases were available. The heterogeneous quality of the published data and missing follow-up details in more than half of the researched cases were the main reasons for the inability to perform a precise statistical evaluation. The lack of laboratory examination results and sparse imaging characteristics did not allow us to obtain a more profound evaluation of the influence of differentiation on prognosis. Chemotherapy with Ewing’s sarcoma-directed protocol and various osteosarcoma-directed protocols could affect the necrosis rate of tumor. Due to the retrospective nature of this study, some prognostic factors were analyzed in small sub-groups, such as necrosis rate of tumor, serum ALP and LDH levels. Thus, the conclusion should be further confirmed. However, our study provided several statistically significant predictive factors in the prognosis of SCOS, which will help obtain a more robust rationale to start a protocol for osteosarcoma. The number of cases and incomplete details limited the possibility of further analysis on this subject, and publication of well-documented case reports is encouraged to enable future explorations.
5. Conclusion
To summarize, our study reported our experience in diagnosing and treating SCOS in detail and discovered the potential role of ALP and LDH in the prognosis of SCOS patients. In the pooled analysis, we detected an increasing trend for the survival rate in SCOS and verified positive margins, poor response to chemotherapy and occurrence of MD as negative predictors for the prognosis of SCOS. However, no imaging features related to prognosis were found. MD and LR were significantly correlated and could arise a dozen years after treatment, supporting the need for prolonged or more intensive surveillance in patients with MD or LR. Further analysis could be possible if more literature reporting response to chemotherapy, laboratory examinations and imaging features in detail, were available in future.
CRediT authorship contribution statement
Jingyu Zhong: Conceptualization, Methodology, Investigation, Writing - original draft, Writing - review & editing. Yangfan Hu: Conceptualization, Methodology, Investigation, Writing - review & editing. Liping Si: Investigation, Writing - review & editing. Jia Geng: Investigation, Writing - review & editing. Yue Xing: Investigation, Writing - review & editing. Qiong Jiao: Investigation, Writing - review & editing. Huizhen Zhang: Investigation, Writing - review & editing, Supervision. Weiwu Yao: Conceptualization, Writing - review & editing, Supervision, Funding acquisition.
Acknowledgments
Acknowledgments
The authors would like to thank Prof. Huan Zhang for her kindness and enlightening comments on this study, Dr. Guangcheng Zhang for support in patients’ follow-up, and Dr. Shiqi Mao for his constructive discussion and suggestions, Mr. Ruixiang Zhao for his assistance in artwork drafting as well as American Journal Experts for English language editing.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81771790] and the Medicine and Engineering Combination Project of Shanghai Jiao Tong University [grant number YG2019ZDB09].
Contributor Information
Huizhen Zhang, Email: liuyuanblz@aliyun.com.
Weiwu Yao, Email: yaoweiwuhuan@163.com.
References
- 1.Sim F.H., Unni K.K., Beabout J.W., Dahlin D.C. Osteosarcoma with small cells simulating Ewing's tumor. J. Bone Joint Surg. Am. 1979;61(2):207–215. [PubMed] [Google Scholar]
- 2.Fletcher C.D.M., Bridge J.A., Hogendoorn P.C.W., Mertens F. Chapter 16 Osteogenic Tumors. 4th ed. IARC Press; Lyon: 2013. World Health Organization classification of tumors. WHO classification of tumours of soft tissue and bone; p. 291. [Google Scholar]
- 3.Kilpatrick S.E., Reith J.D., Rubin B. Ewing sarcoma and the history of similar and possibly related small round cell tumors: from whence have we come and where are we going? Adv. Anat. Pathol. 2018;25(5):314–326. doi: 10.1097/PAP.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 4.Yarmish G., Klein M.J., Landa J., Lefkowitz R.A., Hwang S. Imaging characteristics of primary osteosarcoma: nonconventional subtypes. Radiographics. 2010;30(6):1653–1672. doi: 10.1148/rg.306105524. [DOI] [PubMed] [Google Scholar]
- 5.Klein M.J., Siegal G.P. Osteosarcoma: anatomic and histologic variants. Am. J. Clin. Pathol. 2006;125(4):555–581. doi: 10.1309/UC6K-QHLD-9LV2-KENN. [DOI] [PubMed] [Google Scholar]
- 6.Edeiken J., Raymond A.K., Ayala A.G., Benjamin R.S., Murray J.A., Carrasco H.C. Small-cell osteosarcoma. Skeletal Radiol. 1987;16(8):621–628. doi: 10.1007/BF00357110. [DOI] [PubMed] [Google Scholar]
- 7.Martin S.E., Dwyer A., Kissane J.M., Costa J. Small-cell osteosarcoma. Cancer. 1982;50(5):990–996. doi: 10.1002/1097-0142(19820901)50:5<990::aid-cncr2820500529>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Bertoni F., Present D., Bacchini P., Pignatti G., Picci P., Campanacci M. The Istituto Rizzoli experience with small cell osteosarcoma. Cancer. 1989;64(12):2591–2599. doi: 10.1002/1097-0142(19891215)64:12<2591::aid-cncr2820641231>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Ayala A.G., Ro J.Y., Raymond A.K. Small cell osteosarcoma. A clinicopathologic study of 27 cases. Cancer. 1989;64(10):2162–2173. doi: 10.1002/1097-0142(19891115)64:10<2162::aid-cncr2820641031>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 10.Ayala A.G., Ro J.Y., Papadopoulos N.K., Raymond A.K., Edeiken J. Small Cell Osteosarcoma. Kluwer Academic Publishers; Boston: 1993. Oestosarcoma in adolescents and young adults; pp. 139–149. [Google Scholar]
- 11.Nakajima H., Sim F.H., Bond J.R., Unni K.K. Small cell osteosarcoma of bone. Review of 72 cases. Cancer. 1997;79(11):2095–2106. doi: 10.1002/(sici)1097-0142(19970601)79:11<2095::aid-cncr6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Righi A., Gambarotti M., Longo S. Small cell osteosarcoma: clinicopathologic, immunohistochemical, and molecular analysis of 36 cases. Am. J. Surg. Pathol. 2015;39(5):691–699. doi: 10.1097/PAS.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 13.Bishop J.A., Shum C.H., Sheth S., Wakely P.E., Jr, Ali S.Z. Small cell osteosarcoma: cytopathologic characteristics and differential diagnosis. Am. J. Clin. Pathol. 2010;133(5):756–761. doi: 10.1309/AJCPO07VGDZCBRJF. [DOI] [PubMed] [Google Scholar]
- 14.Machado I., Alberghini M., Giner F. Histopathological characterization of small cell osteosarcoma with immunohistochemistry and molecular genetic support. A study of 10 cases. Histopathology. 2010;57(1):162–167. doi: 10.1111/j.1365-2559.2010.03589.x. [DOI] [PubMed] [Google Scholar]
- 15.Stea B., Cavazzana A., Kinsella T.J. Small-cell osteosarcoma: correlation of in vitro and clinical radiation response. Int. J. Radiat. Oncol. Biol. Phys. 1988;15(5):1233–1238. doi: 10.1016/0360-3016(88)90209-x. [DOI] [PubMed] [Google Scholar]
- 16.Mirabello L., Troisi R.J., Savage S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115(7):1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duchman K.R., Gao Y., Miller B.J. Prognostic factors for survival in patients with high-grade osteosarcoma using the Surveillance, Epidemiology, and End Results (SEER) Program database. Cancer Epidemiol. 2015;39(4):593–599. doi: 10.1016/j.canep.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Enneking W.F., Spanier S.S., Goodman M.A. A system for the surgical staging of musculoskeletal sarcoma. Clin. Orthop. Relat. Res. 1980;153:106–120. [PubMed] [Google Scholar]
- 19.Rosen G., Caparros B., Huvos A.G. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49:1221–1230. doi: 10.1002/1097-0142(19820315)49:6<1221::aid-cncr2820490625>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Giangaspero F., Stracca V., Visonà A., Eusebi V. Small-cell osteosarcoma of the mandible. Case report. Appl. Pathol. 1984;2(1):28–31. [PubMed] [Google Scholar]
- 21.Roessner A., Immenkamp M., Hiddemann W., Althoff J., Miebs T., Grundmann E. Case report 331: Small cell osteosarcoma of the tibia with diffuse metastatic disease. Skeletal Radiol. 1985;14(3):216–225. doi: 10.1007/BF00355568. [DOI] [PubMed] [Google Scholar]
- 22.Sanjay B., Raj G.A., Vishwakarma G. A small-cell osteosarcoma with multiple skeletal metastases. Arch. Orthop. Trauma Surg. 1988;107(1):58–60. doi: 10.1007/BF00463527. [DOI] [PubMed] [Google Scholar]
- 23.Noguera R., Navarro S., Triche T.J. Translocation (11;22) in small cell osteosarcoma. Cancer Genet. Cytogenet. 1990;45(1):121–124. doi: 10.1016/0165-4608(90)90074-k. [DOI] [PubMed] [Google Scholar]
- 24.Dickersin G.R., Rosenberg A.E. The ultrastructure of small-cell osteosarcoma, with a review of the light microscopy and differential diagnosis. Hum. Pathol. 1991;22(3):267–275. doi: 10.1016/0046-8177(91)90161-h. [DOI] [PubMed] [Google Scholar]
- 25.Huang X., Sang F. Small cell osteosarcoma. Chin. J. Clin. Exp. Pathol. 1991;7(2):149–150. [Article in Chinese] [Google Scholar]
- 26.Kyriakos M., Gilula L.A., Becich M.J., Schoenecker P.L. Intracortical small cell osteosarcoma. Clin. Orthop. Relat. Res. 1992;279:269–280. [PubMed] [Google Scholar]
- 27.Devaney K., Vinh T.N., Sweet D.E. Small cell osteosarcoma of bone: an immunohistochemical study with differential diagnostic considerations. Hum. Pathol. 1993;24(11):1211–1225. doi: 10.1016/0046-8177(93)90218-6. [DOI] [PubMed] [Google Scholar]
- 28.Stracca-Pansa V., Dickman P.S., Zamboni G., Bevilacqua P.A., Ninfo V. Extracellular matrix of small round cell tumors of childhood: an immunohistochemical study of 67 cases. Pediatr. Pathol. 1994;14(1):111–125. doi: 10.3109/15513819409022031. [DOI] [PubMed] [Google Scholar]
- 29.Park Y.K., Ryu K.N., Ahn J.H., Yang M.H. A small cell osteosarcoma on the calcaneus–a case report. J. Korean Med. Sci. 1995;10(2):147–151. doi: 10.3346/jkms.1995.10.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson L.H., Pitt M.J., Jaffe K.A., Siegal G.P. Small cell osteosarcoma of the soft tissue. Skeletal Radiol. 1995;24(6):462–465. doi: 10.1007/BF00941249. [DOI] [PubMed] [Google Scholar]
- 31.Sipos E.P., Tamargo R.J., Epstein J.I., North R.B. Primary intracerebral small-cell osteosarcoma in an adolescent girl: report of a case. J. Neurooncol. 1997;32(2):169–174. doi: 10.1023/a:1005775818317. [DOI] [PubMed] [Google Scholar]
- 32.Ye X. Periosteal small cell osteosarcoma: a case report. J. Diag. Pathl. 1997;4(2):74. . [Article in Chinese] [Google Scholar]
- 33.Tian Y., An H. CT feature of clavicle small cell osteosarcoma. Mod. Med. Imag. 1998;7(6):271. [Article in Chinese] [Google Scholar]
- 34.Lintz K.C., Penson R.T., Cassem N., Harmon D.C., Chabner B.A., Lynch T.J. A staff dialogue on aggressive palliative treatment demanded by a terminally ill patient: psychosocial issues faced by patients, their families, and caregivers. Oncologist. 1999;4(1):70–76. [PubMed] [Google Scholar]
- 35.Mulligan M.E., Lewis D.R., Jr, Resnik C.S., Kumar D., Levine A.M. Small cell osteosarcoma of the ulna: a case report and review of the literature. J. Hand Surg. Am. 1999;24(2):417–420. doi: 10.1053/jhsu.1999.0417. [DOI] [PubMed] [Google Scholar]
- 36.Park S.H., Kim I. Small cell osteogenic sarcoma of the ribs: cytological, immunohistochemical, and ultrastructural study with literature review. Ultrastruct. Pathol. 1999;23(2):133–140. doi: 10.1080/019131299281770. [DOI] [PubMed] [Google Scholar]
- 37.Todesco A., Carli M., Iacona I., Frascella E., Ninfo V., Rosolen A. All-trans retinoic acid and interferon-alpha in the treatment of a patient with resistant metastatic osteosarcoma. Cancer. 2000;89(12):2661–2666. doi: 10.1002/1097-0142(20001215)89:12<2661::aid-cncr20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura T., Ishimaru J., Mizui T. Osteosarcoma metastatic to the mandible: a case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001;91(4):452–454. doi: 10.1067/moe.2001.113107. [DOI] [PubMed] [Google Scholar]
- 39.Cai R., Zhou J. Small cell osteosarcoma: a case report. J. Clin. Exp. Pathol. 2002;18(4):457–458. [Article in Chinese] [Google Scholar]
- 40.Zhang X., Yao F., Liu Y., Liu L. Small cell osteosarcoma of tibia: a case report. J. Med. Imaging. 2003;13(12):889–893. [Article in Chinese] [Google Scholar]
- 41.Nishio J., Gentry J.D., Neff J.R. Monoallelic deletion of the p53 gene through chromosomal translocation in a small cell osteosarcoma. Virchows Arch. 2006;448(6):852–856. doi: 10.1007/s00428-006-0181-x. [DOI] [PubMed] [Google Scholar]
- 42.Weng Y., Niu H., Deng Z. Extraskeletal small cell osteosarcoma in leg:a clinicopathological analysis. J. Diag. Pathol. 2006;13(4):278–280. [Article in Chinese] [Google Scholar]
- 43.Fang D., Ma F. Small cell osteosarcoma of mandible: a case report. Shandong Med. J. 2009;49(35):64. [Article in Chinese] [Google Scholar]
- 44.Li W., Ge L. Small cell osteosarcoma of ilium: a case report. J. Pract. Med. 2009;25(18):3005. [Article in Chinese] [Google Scholar]
- 45.Kallel R., Ayadi L., Toumi N. Small cell osteosarcoma: a case report. Pathologica. 2009;101(2):101–104. [PubMed] [Google Scholar]
- 46.Yang J.Y., Kim J.M. Small cell extraskeletal osteosarcoma. Orthopedics. 2009;32(3):217. [PubMed] [Google Scholar]
- 47.Posthumadeboer J., Graat H.C., Bras J., Saouti R. Small cell osteosarcoma of a toe phalanx: a case report and review of literature. J. Orthop. Surg. Res. 2010;5:36. doi: 10.1186/1749-799X-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi L., Wang R., Wang C. Small cell osteosarcoma of petrous apex. Radiol. Practice. 2010;25(5):587–588. [Article in Chinese] [Google Scholar]
- 49.Debelenko L.V., McGregor L.M., Shivakumar B.R., Dorfman H.D., Raimondi S.C. A novel EWSR1-CREB3L1 fusion transcript in a case of small cell osteosarcoma. Genes Chromosom. Cancer. 2011;50(12):1054–1062. doi: 10.1002/gcc.20923. [DOI] [PubMed] [Google Scholar]
- 50.Uma K., Cherian G., Nayak V., Patil S. Small cell osteosarcoma of the mandible: Case report and review of its diagnostic aspects. J. Oral Maxillofac. Pathol. 2011;15(3):330–334. doi: 10.4103/0973-029X.86713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ali R.H., Lee C.H., Hayes M.M. Metastatic small cell osteosarcoma to the liver: a diagnostic pitfall for fine-needle aspiration cytology. Diagn. Cytopathol. 2014;42(2):161–164. doi: 10.1002/dc.22894. [DOI] [PubMed] [Google Scholar]
- 52.Fındık G., Günay E., Ağaçkıran Y., Aydoğdu K., Aydın E., Kaya S. Small cell osteosarcoma of rib: diagnosis and treatment of the rare case. Tuberk Toraks. 2012;60(2):172–175. [PubMed] [Google Scholar]
- 53.Futani H., Fukunaga S., Tsukamoto Y. Small cell osteosarcoma successfully treated by high-dose ifosfamide and methotrexate, combined with carboplatin and pirarubicin. Anticancer Res. 2012;32(3):965–971. [PubMed] [Google Scholar]
- 54.Huang W., Zhang H., Jiang Z. Small cell osteosarcoma: a clinicopathological analysis of 14 cases. J. Clin. Exp. Pathol. 2012;28(6):643–646. [Article in Chinese] [Google Scholar]
- 55.Jambhekar N.A., Agarwal M., Suryawanshi P. Osteosarcoma of the femur mimicking Ewing sarcoma/primitive neuroectodermal tumour on biopsy and metastatic carcinoma on resection. Skeletal Radiol. 2012;41(9):1163–1168. doi: 10.1007/s00256-012-1406-5. [DOI] [PubMed] [Google Scholar]
- 56.Pugi A., Benemei S., Vietri M. Anaphylaxis during the first course of high-dose methotrexate: a case report and literature review. J. Clin. Pharm. Ther. 2012;37(2):245–248. doi: 10.1111/j.1365-2710.2011.01275.x. [DOI] [PubMed] [Google Scholar]
- 57.Kang K., Lee J.H., Kim H.G. Contralateral referred pain in a patient with intramedullary spinal cord metastasis from extraskeletal small cell osteosarcoma. J. Spinal Cord Med. 2013;36(6):695–699. doi: 10.1179/2045772312Y.0000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wan X., Zhou L. Clinical Features and Treatment of Small Cell Osteosarcoma: A Report of Twelve Cases and Literature Review. West China Medical Journal. 2013;28(5):715–718. [Article in Chinese] [Google Scholar]
- 59.Sood N., Rewri S., Nigam J.S. Small cell extraskeletal osteosarcoma: a rare case report. Rare Tumors. 2014;6(1):5029. doi: 10.4081/rt.2014.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang M., Zhang W., Li Q., Qu J.L., Zhang G.F. Small-cell extraskeletal osteosarcoma: case report and literature review. Int J Clin Exp Pathol. 2014;7(2):797–800. [PMC free article] [PubMed] [Google Scholar]
- 61.Agarwal S., Gahlot G.P., Bhalla A., Bakhshi S. Small cell osteosarcoma of the parietal region: a unique case at an unusual site. BMJ Case Rep. 2015 doi: 10.1136/bcr-2015-210086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Handa U., Mundi I., Mohan H., Garg S.K. Cytological diagnosis of small cell osteosarcoma of the bone. J. Cytol. 2015;32(2):136–138. doi: 10.4103/0970-9371.160573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen C., Cheng J., Zhang Y., Wang W. MR features of primary intraspinal small cell extraskeletal osteosarcoma: Case report. Chin. J. Med. Imaging Techn. 2017;33(6):963–964. [Article in Chinese] [Google Scholar]
- 64.Jeong H.I., Lee M.J., Nam W., Cha I.H., Kim H.J. Osteosarcoma of the Jaws in Koreans: Analysis of 26 Cases. J. Korean Assoc. Oral Maxillofac. Surg. 2017;43(5):312–317. doi: 10.5125/jkaoms.2017.43.5.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X. Clinicopathological anaylsis of small cell osteosarcoma. World Clin. Med. 2017;11(6):67–71. [Article in Chinese] [Google Scholar]
- 66.AbdullGaffar B., Keloth T. Laryngeal sarcomas: A case series of 5 cases. Ann. Diagn. Pathol. 2018;37:35–41. doi: 10.1016/j.anndiagpath.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 67.Syriac K.A., Bhaskarla A.V., Elrifai M., Alraiyes A.H. Endobronchial metastasis as an uncommon pattern of metastatic dissemination from small cell osteosarcoma. BMJ Case Rep. 2019;12(7) doi: 10.1136/bcr-2019-229779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin F., Wang Q., Yu W. Clinical analysis of Chinese limb osteosarcoma patients treated by two combinations of methotrexate, cisplatin, doxorubicin and ifosfamide. Asia Pac. J. Clin. Oncol. 2011;7(3):270–275. doi: 10.1111/j.1743-7563.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 69.Zheng S., Zhou S., Qiao G. Pirarubicin-based chemotherapy displayed better clinical outcomes and lower toxicity than did doxorubicin-based chemotherapy in the treatment of non-metastatic extremity osteosarcoma. Am. J. Cancer Res. 2014;5(1):411–422. [PMC free article] [PubMed] [Google Scholar]
- 70.Grier H.E., Krailo M.D., Tarbell N.J. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N. Engl. J. Med. 2003;348(8):694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 71.Bielack S.S., Kempf-Bielack B., Delling G. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002;20(3):776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 72.Bacci G., Longhi A., Ferrari S. Prognostic significance of serum lactate dehydrogenase in osteosarcoma of the extremity: experience at Rizzoli on 1421 patients treated over the last 30 years. Tumori. 2004;90(5):478–484. doi: 10.1177/030089160409000507. [DOI] [PubMed] [Google Scholar]
- 73.Bacci G., Longhi A., Versari M., Mercuri M., Briccoli A., Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106(5):1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 74.Hao H., Chen L., Huang D., Ge J., Qiu Y., Hao L. Meta-analysis of alkaline phosphatase and prognosis for osteosarcoma. Eur. J. Cancer Care (Engl). 2017;26(5) doi: 10.1111/ecc.12536. [DOI] [PubMed] [Google Scholar]
- 75.Fu Y., Lan T., Cai H., Lu A., Yu W. Meta-analysis of serum lactate dehydrogenase and prognosis for osteosarcoma. Medicine (Baltimore). 2018;97(19) doi: 10.1097/MD.0000000000010741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kelley L.M., Schlegel M., Hecker-Nolting S. Pathological fracture and prognosis of high-grade osteosarcoma of the extremities: an analysis of 2,847 consecutive cooperative osteosarcoma study group (COSS) patients. J. Clin. Oncol. 2020;38(8):823–833. doi: 10.1200/JCO.19.00827. [DOI] [PubMed] [Google Scholar]