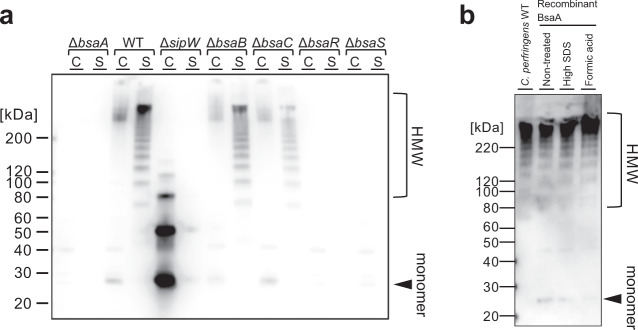
Fig. 2. Polymerization of extracellularly secreted BsaA protein.
a Western blotting of BsaA. Cell extracts (C) and culture supernatants (S) were isolated from WT or mutant strains grown to the mid-exponential phase (OD600 = 1.0) at 25 °C. Protein samples (OD600 unit = 0.01) were separated on a 4–12% gradient SDS-polyacrylamide gel. BsaA proteins were detected with anti-BsaA antisera. b Recombinant BsaA proteins also form polymers. Recombinant BsaA-6×His proteins were purified by affinity binding to Ni-NTA affinity resin from E. coli. BsaA-6×His proteins were incubated in 10% SDS for 10 min at 95 °C or in 20% formic acid for 10 min at the room temperature. Protein samples of C. perfringens WT cells (OD600 unit = 0.004) and purified BsaA-6×His proteins (10 ng) were separated on a 4–12% gradient SDS-polyacrylamide gel. BsaA proteins were detected by western blotting with anti-BsaA antisera. HMW protein polymers and protein monomers are shown.