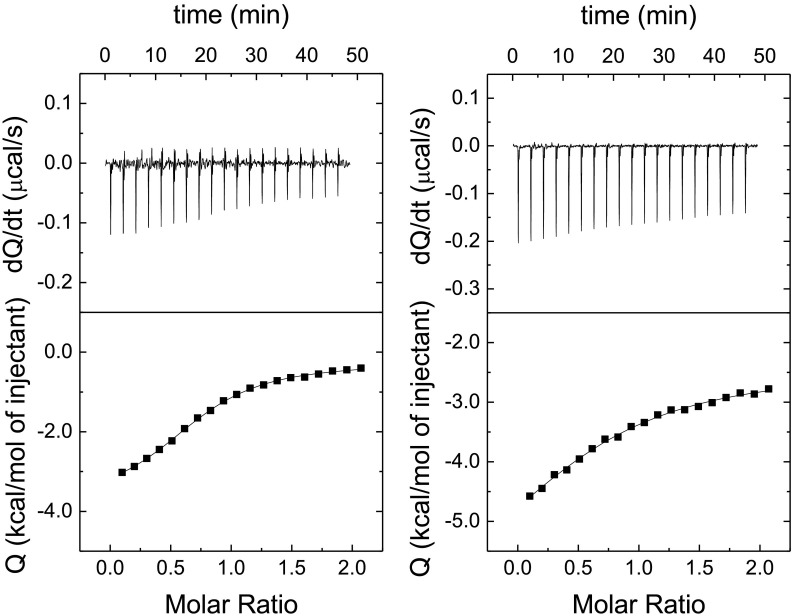
Fig. 8.
Hit conformation through isothermal titration calorimetry (ITC). Interaction of quercetin with 3CLpro assessed by isothermal titration calorimetry in sodium phosphate 50 mM, pH 8, without (left) and with (right) NaCl 150 mM. The upper plot shows the thermogram (thermal power required to maintain a null temperature difference between sample and reference cells as a function of time) and the lower plot shows the binding isotherm (ligand-normalized heat effect per injection as a function of the molar ratio, the quotient between the ligand and protein concentrations in the cell). The fitting curve corresponds to the single ligand binding site model (continuous line). According to the data analysis, in the absence of NaCl quercetin interacts with 3CLpro with favorable enthalpic (ΔH = −3.6 kcal/mol) and entropic (−TΔS = −4.0 kcal/mol) contributions to the Gibbs energy of binding (ΔG = −7.6 kcal/mol), corresponding to a dissociation constant Kd of 2.7 μM. In the presence of NaCl quercetin interacts with 3CLpro with favorable enthalpic (ΔH = −4.3 kcal/mol) and entropic (−TΔS = −2.5 kcal/mol) contributions to the Gibbs energy of binding (ΔG = −6.8 kcal/mol), corresponding to a dissociation constant Kd of 10 μM. In both cases, the percentage of active (or binding-competent) protein is 0.75.