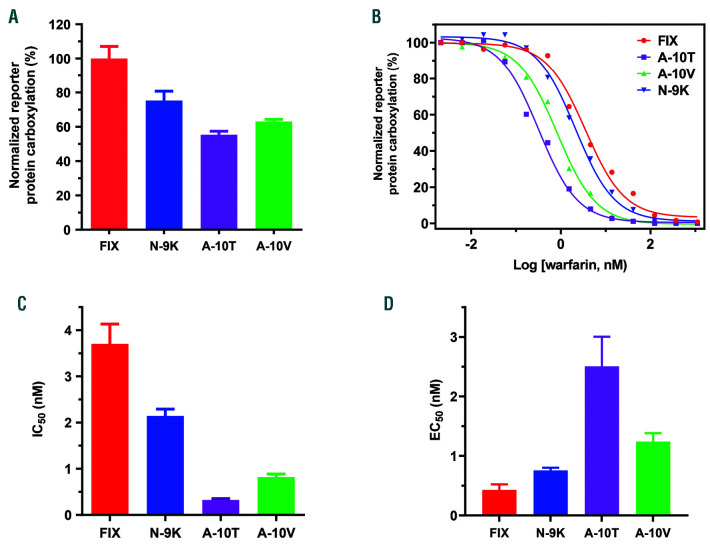
Figure 4.
Effect of naturally occurring propeptide mutations on reporter-protein carboxylation. (A) Carboxylation efficiency of the chimeric reporter-protein with wild-type factor IX (FIX) propeptide or with its naturally occurring mutations N-9K, A-10T, and A-10V. The wild-type and corresponding mutant reporter-proteins were transiently expressed in HEK293 cells and the carboxylated reporter-protein was determined by ELISA, as described in the legend to Figure 1C. (B) Warfarin titration of reporter-protein carboxylation as directed by FIX propeptide with naturally occurring mutations at positions −9 and −10. The wild-type and corresponding mutant reporter-proteins were stably expressed in HEK293 cells. The corresponding reporter cells were cultured in complete medium containing 20 nM vitamin K with increasing concentrations of warfarin. (C) Inhibition efficiency of warfarin on the carboxylation of mutant reporter-proteins. The half-maximal inhibition concentration (IC50) of warfarin was determined from (B) using GraphPad software. (D) Effect of vitamin K concentration on reporter-protein carboxylation. HEK 293 cells stably expressing wild-type and corresponding mutant reporter-proteins were cultured with complete medium containing increasing concentrations of vitamin K. The efficiency of reporter-protein carboxylation was determined by ELISA and the half-maximal effective concentration (EC50) of vitamin K was determined using GraphPad software.