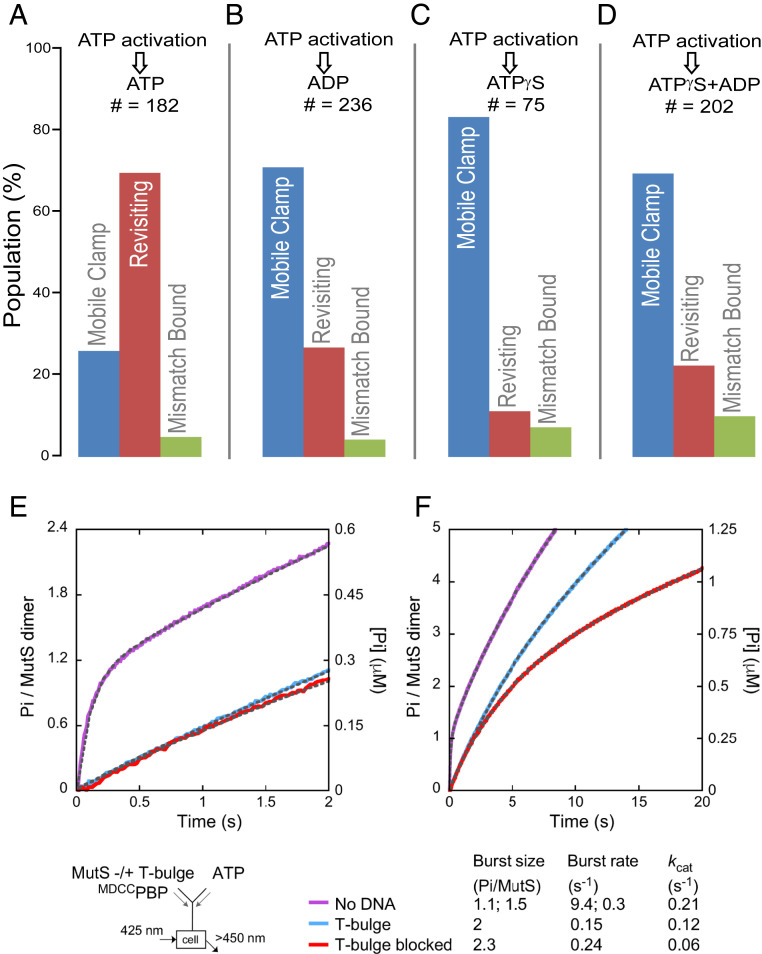
Fig. 2.
Impact of nucleotide exchange on MutS revisiting a mismatch on end-blocked DNA. (A–D) Exchanging buffer after mobile clamp formation (second buffer exchange, Fig. 1E) to introduce ADP (B), ATP-γ-S (C), or ADP+ATP-γ-S (D) instead of ATP (A) significantly reduces the MutS mobile clamp population revisiting the mismatch. Color bars and state descriptions are as defined in Fig. 1F. (E and F) Presteady-state ATPase kinetics of free MutS (purple trace), and mixed with unblocked DNA (blue trace) or doubly end-blocked DNA (red trace) shown at 2-s (E) and 20-s (F) time scales (SI Appendix, Supporting Methods). Exponential+linear fits to the data are overlaid on the traces as dashed lines (amplitudes and rate constants listed below the graph; residuals in SI Appendix, Fig. S3). MutS alone shows burst hydrolysis of one ATP/dimer at k = 9.4 ± 0.2 s−1, the second ATP/dimer at k = 0.3 ± 0.02 s−1, and then a linear, steady state kcat = 0.21 ± 0.06 s−1 (purple trace). On unblocked T-bulge DNA, MutS shows a slow burst of two ATP/dimer hydrolyzed at k = 0.15 ± 0.03 s−1, followed by kcat = 0.12 ± 0.02 s−1 (blue trace). On doubly end-blocked T-bulge DNA, MutS again shows a burst of two ATP/dimer hydrolyzed at k = 0.24 ± 0.007 s−1, followed by kcat = 0.06 ± 0.004 s−1 (red trace).