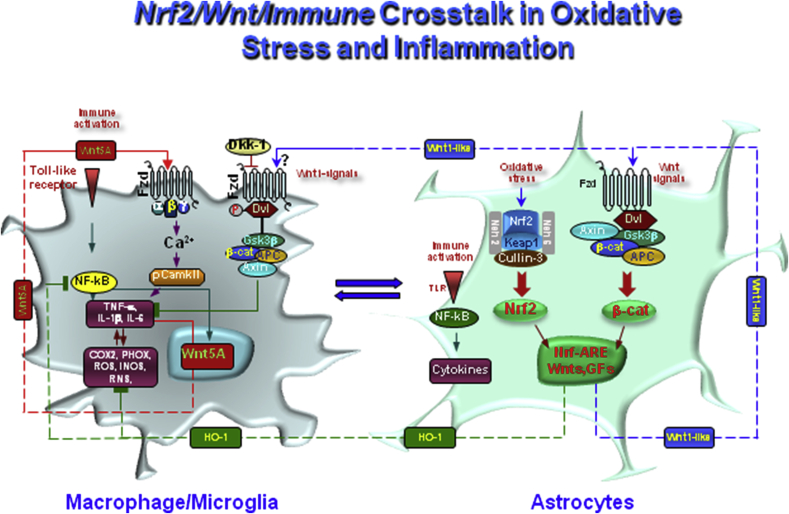
Fig. 6.
Nrf2/Wnt/immune crosstalk in oxidative stress and inflammation in PD. Schematic illustration of astrocyte-microglia crosstalk. Upon activation by neurotoxins, endotoxins, brain injury and ageing, macrophage/microglia produce a panel of pro-inflammatory cytokines (TNF-α and IL-1β) and chemokines (CCL3, CXCl10 and CXCL11). Up-regulation of microglial PHOX-derived ROS, iNOS-derived NO, and GSK-3β, a known regulator of NF-kB-dependent gene transcription, further exacerbates microglia reaction. Wnt5a constitutes one part of a self-perpetrating cycle, via autocrine Wnt5A/CamKII activation and paracrine stimulation of Th-1- cytokines, iNOS and COX2 [280–2282]. To restrain microglia exacerbation, up-regulation of astrocyte- Nrf2/HO-1 and Wnt1/β-catenin, mitigate the inflammatory milieu and favor a down-regulation of cytokines expression. NF-κB and the Wnt/β-catenin pathway also interact to differentially regulate inflammation, with GSK-3β playing a central role in between. While GSK-3β is a negative regulator of β-catenin, it positively regulates NF-κB by targeting IkB, the major inhibitor of NF-κB, to proteasomal degradation. On the other hand, β-catenin itself can form a complex with the p50 subunit of NF-κB, thereby preventing NF-κB transcriptional activity. Besides, HO-1 indirect modulation, Nrf2-NF-κB interplay contributes to the regulation of immune response under oxidative stress and inflammation aimed at counterbalancing the exacerbated inflammation. Then, astrocyte upregulation of Nrf2/HO-1 and Wnt1/β-catenin during oxidative stress and inflammation represent a critical regulatory level, whereby astrocytes can mitigate M1 exacerbated phenotype and the heighthened levels of proinflammatory cytokines.