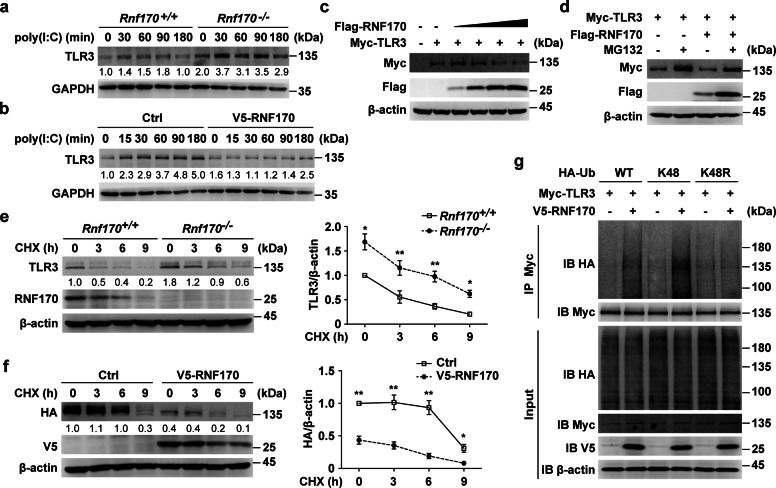
Fig. 6.
RNF170 promotes the K48-linked ubiquitination and proteasomal degradation of TLR3. a Rnf170+/+ and Rnf170−/− RAW264.7 cells were stimulated with poly(I:C) for the indicated time. Cell lysates were immunoblotted with the indicated antibodies. b RAW264.7 cells stably expressing V5-RNF170 were stimulated with poly(I:C) for the indicated time. Cell lysates were immunoblotted with the indicated antibodies. c HEK293T cells were transfected with a Myc-TLR3 plasmid and increasing amounts of Flag-RNF170 for 24 h. Cell lysates were immunoblotted with the indicated antibodies. d HEK293T cells were transfected with the indicated plasmids for 24 h and treated with MG132 (3 μM) for 7 h. Cell lysates were immunoblotted with the indicated antibodies. e Rnf170+/+ and Rnf170−/− RAW264.7 cells were stimulated with poly(I:C) for 1.5 h and then treated with cycloheximide (CHX) for the indicated times, and then cell lysates were immunoblotted with the indicated antibodies. The image intensity of TLR3 was quantified on the right. f L929 cells were transfected with the indicated plasmids for 36 h, stimulated with poly(I:C) for 9 h and then treated with CHX for the indicated times. Cell lysates were immunoblotted with the indicated antibodies. The image intensity of HA-TLR3 was quantified on the right. g HEK293T cells were transfected with the indicated plasmids for 24 h and treated with MG132 (3 μM) for 7 h. Cell lysates were immunoprecipitated using anti-Myc antibodies. The precipitates were analyzed by immunoblot analysis using the indicated antibodies. Data are representative of three independent experiments (a–g). Data are shown as the mean ± SEM in e and f (n = 3). *P < 0.05 and **P < 0.01; paired Student’s t-test