Table 1.
Inhibition activities of isatin derivatives against SARS-CoV-2 3CLpro.
| Compds | R1 | R2 | IC50 or percentage (%) of inhibition at 50 μMb |
|---|---|---|---|
|
Tideglusiba |
1.91 ± 0.16 |
||
| 1 |  |
H | -c |
| 2 | -CH2OH | H | – |
| 3 | 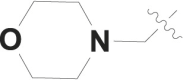 |
F | – |
| 4 | 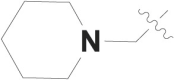 |
F | – |
| 5 | 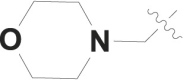 |
Cl | – |
| 6 |  |
NO2 | – |
| 7 | CH3 | I | 25% at 50 μM |
| 8 | CH3CH2CH2 | I | 51% at 50 μM |
| 9 | n-C4H9 | I | 41.8 ± 8.0 |
| 10 | CH3 | CO2CH3 | 53% at 50 μM |
| 11 | CH3CH2CH2 | CO2CH3 | 53% at 50 μM |
| 12 | n-C4H9 | CO2CH3 | 42% at 50 μM |
| 13 | 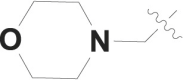 |
CO2CH3 | 32% at 50 μM |
| 14 | PhCH2 | CO2CH3 | 45% at 50 μM |
| 15 | CH3 | 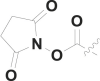 |
45% at 50 μM |
| 16 | n-C4H9 | 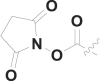 |
47% at 50 μM |
| 17 | PhCH2 | 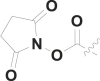 |
15.5 ± 1.2 |
| 18 | CH3 | CO2H | – |
| 19 | CH3CH2CH2 | CO2H | – |
| 20 | n-C4H9 | CO2H | – |
| 21 | PhCH2 | CO2H | – |
| 22 | β-C10H7CH2 | CO2H | 32% at 50 μM |
| 23 | 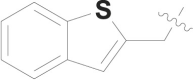 |
CONH2 | 0.053 ± 0.010 |
| 24 | CH3CH2CH2 | CONH2 | 10.2 ± 1.0 |
| 25 | n-C4H9 | CONH2 | 17.8 ± 0.7 |
| 26 | β-C10H7CH2 | CONH2 | 0.045 ± 0.007 |
| 27 | β-(6-Br)-C10H7CH2 | CONH2 | 0.047 ± 0.007 |
| 28 | β-(6-Ph)-C10H7CH2 | CONH2 | 24.9 ± 4.6 |
| 29 | β-C10H7CH2 | CH2CONH2 | 39.2 ± 10.5 |

