Scheme 1.
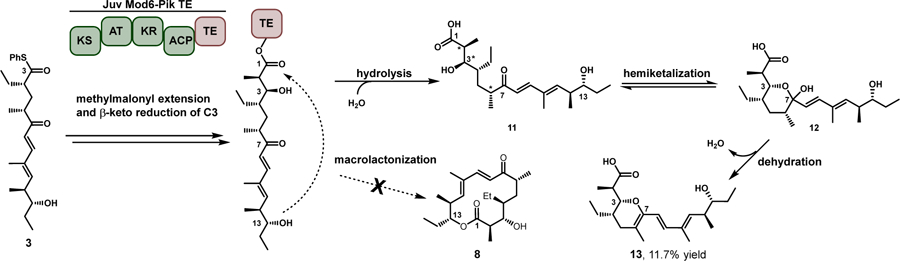
Reaction of Juv Mod6-Pik TE with Tyl hexaketide 3 results in pyran 13, which is identical in mass to macrolactone 8. This outcome was only achieved via extension of Tyl hexaketide through Juv Mod6-Pik TE and hydrolytic offloading to the linear seco acid 11, which can rapidly interconvert to the hemiketal containing product 12. Finally, the hemiketal is spontaneously dehydrated to produce the observed pyran 13. Stereochemistry displayed is that of the native product 11 and subsequent hemiketalization (12). *represent likely points of inverted stereochemistry seen in the isolated product.
