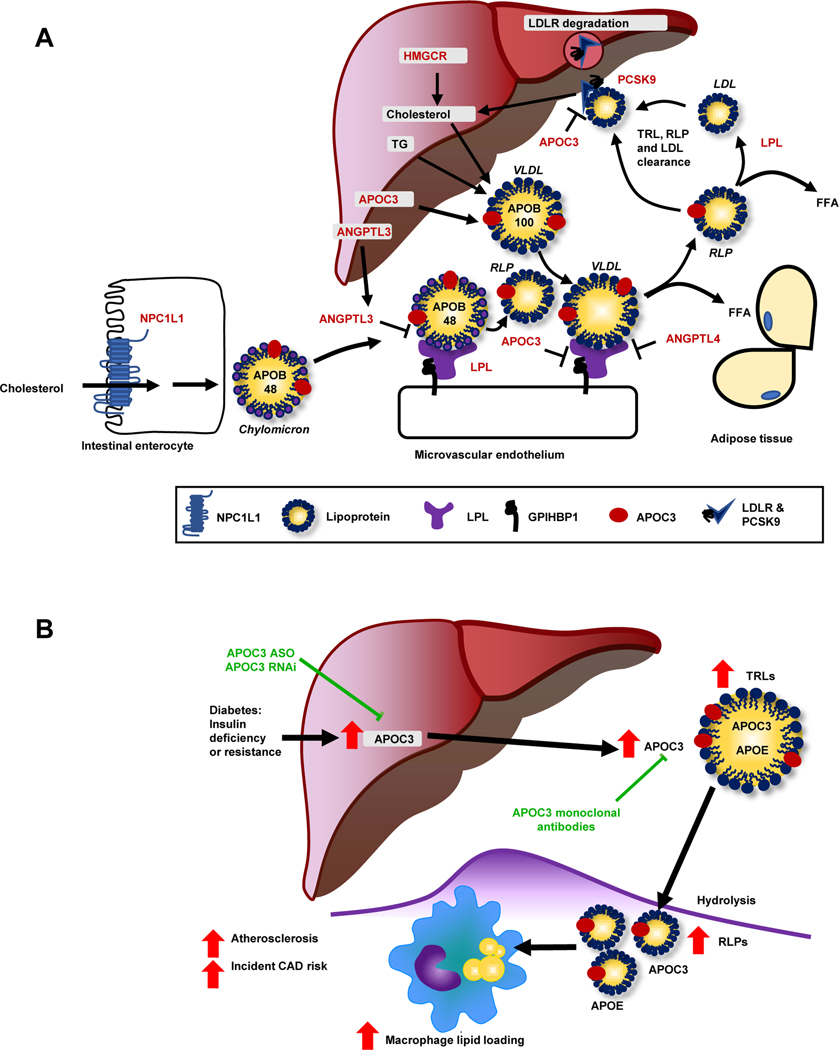
Figure 2. Lipoprotein metabolism at a glance.
(A) This schematic shows the genes discussed in relation to lipid metabolism (highlighted by red font). Cholesterol and sterols are taken up from the intestinal lumen via the Nieman Pick C1-like 1 (NPC1L1) transporter (blue, lower left corner) and are then re-assembled into chylomicrons containing apolipoprotein (APO) B48. The liver produces cholesterol through HMG-CoA reductase (HMGCR; upper center), which is incorporated with triglycerides into APOB100-containing VLDL. LPL (purple) hydrolyzes triglycerides into free fatty acids (FFA), resulting in the formation of LDL and RLPs from VLDL and chylomicrons, respectively, which can enter tissues. Most APOB-containing lipoproteins are taken up via the hepatic LDL receptor (LDLR). Proprotein convertase subtilisin/kexin type 9 (PCSK9) stimulates internalization and degradation of the LDLR, thereby reducing cell surface expression of the LDLR. Angiopoietin-like (ANGPL) 3, ANGPTL4, and APOC3 are endogenous inhibitors of LPL. APOC3 can also suppress hepatic uptake of APOEcontaining lipoprotein particles (found e.g., on VLDL and RLPs). (B) Diabetes enhances hepatic production of APOC3. Elevated levels of APOC3 increase circulating levels of triglyceride-rich lipoproteins (TRLs), resulting in accumulation of RLPs in the artery wall and accelerated atherosclerosis, in part via increased macrophage lipid loading. An antisense oligonucleotide (ASO) to APOC3 completely blocks diabetes-accelerated atherosclerosis in a mouse model. Other emerging strategies to block APOC3 include small interfering RNA (RNAi) and monoclonal antibodies. Figure 2B is reproduced and modified from [80] with permission from the Journal of Clinical Investigation.