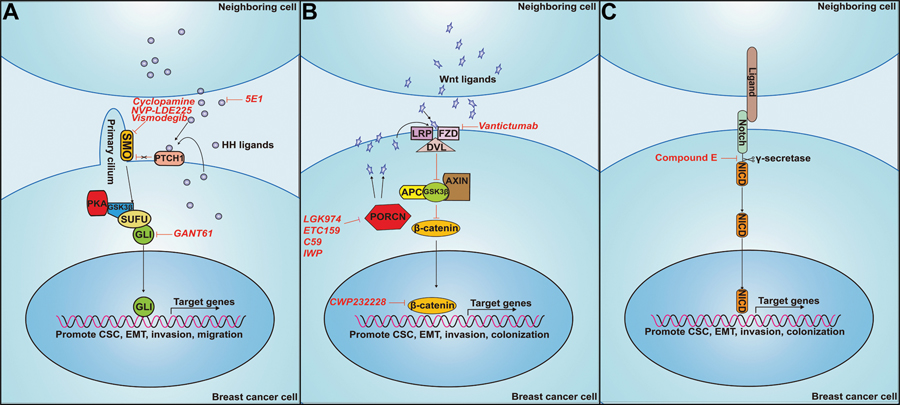
Figure 1: Means to inhibit canonical HH, WNT and NOTCH pathways.
A. HH Pathway. Binding of HH ligands (SHH, DHH or IHH) to PTCH receptors relieves PTCH-mediated inhibition of SMO. SMO inhibits PKA, GSK3β, and SUFU from binding to GLI, allowing for stabilization of the activator confirmation, translocation to the nucleus and activation of HH target genes. The monoclonal antibody, 5E1, binds to SHH and prevents its binding to PTCH receptors. Cyclopamine binds to, and prevents, SMO-mediated activation of GLI transcription factors. GANT61 reduces GLI transcription factor DNA binding to block GLI-mediated HH target gene expression. B. WNT pathway. Binding of WNT ligands to the receptor FZD results in translocation of DVL to the membrane. DVL binds to the APC/GSK3β/AXIN complex, leading to its dissociation from β-CATENIN. β-CATENIN is released and stabilized, allowing for translocation to the nucleus, where it activates transcription of target genes. The antibody Vantictumab binds to FZD and prevents WNT ligand binding. LGK974, ETC159, C59 and IWP inhibit PORCN-mediated processing and secretion of WNT ligands that activate the canonical pathway. C. Notch pathway. During canonical signaling, NOTCH receptors on one cell bind to ligand receptors on another cell, after which the NOTCH receptor is cleaved by the protease complex, γ-SECRETASE, leading to release of the NOTCH Intracellular Domain (NOTCH-ICD). NOTCH-ICD translocates to the nucleus to activate transcription of target genes. Compound E functions to block the cleavage of both γ-secretase and NOTCH-ICD, preventing NOTCH-ICD translocation to the nucleus.