Abstract
Aging impairs the regenerative potential of hematopoietic stem cells (HSC) and skews differentiation towards the myeloid lineage. The bone marrow (BM) microenvironment has recently been suggested to influence HSC aging, however the mechanisms whereby BM stromal cells mediate this effect is unknown. Here we show that aging-associated decreased expression of CXCR4 expression on BM mesenchymal stem cells (MSC) plays a crucial role in the development of the hematopoietic stem and progenitor cells (HSPC) aging phenotype. The BM MSC from old mice was sufficient to drive a premature aging phenotype of young HSPC when cultured together ex vivo. The impaired ability of old MSC to support HSPC function is associated with reduced expression of CXCR4 on BM MSC of old mice. Deletion of the CXCR4 gene in young MSC accelerates an aging phenotype in these cells characterized by increased production of reactive oxygen species (ROS), DNA damage, senescence, and reduced proliferation. Culture of HSPC from young mice with CXCR4 deficient MSC also from young mice led to a premature aging phenotype in the young HSPC, as evidenced by reduced hematopoietic regeneration and enhanced myeloid differentiation. Mechanistically, CXCR4 signaling prevents BM MSC dysfunction by suppressing oxidative stress, as treatment of old or CXCR4 deficient MSC with N-acetyl-L-cysteine (NAC), improved their niche supporting activity, and attenuated the HSPC aging phenotype. Our studies suggest that age-associated reduction in CXCR4 expression on BM MSC impairs hematopoietic niche activity with increased ROS production, driving an HSC aging phenotype. Thus, modulation of the SDF-1/CXCR4 axis in MSC may lead to novel interventions to alleviate the age-associated decline in immune/hematopoietic function.
Keywords: HSPC, aging, MSC, CXCR4 and ROS
INTRODUCTION
Aging-associated changes in the hematopoietic system lead to increased incidence of anemia [1], susceptibility to infectious diseases [2, 3], and hematologic malignancies [4, 5]. All blood and immune cells of the hematopoietic system are produced by hematopoietic stem cells (HSC) that reside in highly specialized bone marrow (BM) microenvironment/niches, where HSC intrinsic programs and extrinsic signals from the niche cells regulate their function. Growing evidences suggest that aging-associated defects in the hematopoietic system are a consequence of declining HSC function. Aging is associated with the reduction in HSC regenerative potential and skewed differentiation towards the myeloid lineage [6–9]. Several HSC intrinsic mechanisms that regulate metabolism, replication and DNA damage and repair responses, have been implicated in HSC aging. Recent studies reveal that aging substantially alters the cellular composition of the BM microenvironment, suggesting that aging-related alternation in extrinsic factors may contribute to age-related defects in HSC function. However, factors/mechanisms regulating aging-related defects in the BM microenvironment are not understood.
Mesenchymal stem cells (MSC) form a crucial component of the hematopoietic niche, promoting HSC quiescence, maintenance, and retention in BM [10, 11]. Depletion of niche MSC triggers HSC egress from the BM and impairs hematopoietic reconstitution after genotoxic stress. In our search for extrinsic mechanism regulating HSC activities, we recently found that BM microenvironment/niche stromal cell expression of the chemokine receptor CXCR4 is critical for maintenance of HSC during steady-state, and for hematopoietic regeneration after myeloablation [12]. However, whether the impairment of the CXCR4/SDF-1 axis in the aged bone marrow contributes to loss of HSC regenerative potential is unknown.
In this study, we evaluated the impact of aging on BM MSC. Unexpectedly, we found that CXCR4 expression on BM MSC decreases substantially with age, resulting in increased ROS production and impaired BM niche activity that drives an HSC aging phenotype. These findings suggest that modulation of the CXCR4/SDF-1 axis in MSC may lead to novel intervention to alleviate stem cell aging, thereby improving the age-associated decline in immune/hematopoietic function.
MATERIALS AND METHODS
Mice
Young C57BL/6 and B6.Cg-Tg(CAG-cre/Esr1)5Amc (Tamoxifen–inducible Cre) mice, 2–3 months of age, were purchased from Jackson Laboratories (Bar Harbor, ME). B6.SJL-Ptprca/Pepcb (BoyJ) mice were bred and maintained in the IUSM LARC facility. Old C57BL/6 mice, 20–24 months of age, were obtained from the National Institute of Aging (NIA), or by aging young mice (C57BL/6J; Jackson Laboratories) in our facility for 24 months. CXCR4 floxed mice (CXCR4fl/fl) were originally obtained from Dr. Yong-Rui Zou (Feinstein Institute for Medical Research, Manhasset, NY) and maintained in our animal facility. Global CXCR4 conditional KO mice were generated by crossing CXCR4fl/fl to tamoxifen-inducible Cre+ transgenic mice as we described [12]. CXCR4 gene deletion in 2–3 month old mice was induced by administering tamoxifen (2 mg/mouse, i.p.) for five consecutive days, and these mice were used for HSPC and stromal cell analysis six months post CXCR4 tamoxifen treatment. Hereafter, we refer to CXCR4fl/fl mice as WT, and after tamoxifen treatment as CXCR4 KO mice. All animal experiments were approved by the IUSM IACUC.
Reagents
Antibodies against c-kit (clone: 2B8), Sca-1 (clone: D7), lineage (clone:17A2/RB6–8C5/RA36B2/Ter-119/M1/70), CD48 (clone: HM48–1), CD45 (clone: 30-F11), anti-Ter119 (clone: ter-119) PDGFR (clone: APB5), CD51 (clone: RMV-7) and CD31 (clone: MEC13.3) were from BioLegend (San Diego, CA). Antibodies against CD150 (clone: mShad150), and VE-cadherin (clone: eBioBV13) were from BD Biosciences (San Diego, CA). Anti-Nestin (clone: 307501) antibody was from R&D Systems (Minneapolis, MN) and antibody against KAP1 (clone: 2B11) was purchased from Thermo Fisher Scientific (Grand Island, NY). CellROX and Mito Tracker reagents were from Invitrogen (Waltham, MA). N-acetyl-L-cysteine (NAC) was purchased from Millipore Sigma (St. Louis, MO). CD45 and Ter119 depletion kits were from Miltenyi Biotech (San Diego, CA). Mesencult expansion kit and StemSpan™ medium were purchased from STEMCELL Technologies (Vancouver, Canada). Mouse methylcellulose complete media was from R &D systems (Minneapolis, MN).
Flow-cytometry analysis
Whole BM cells were treated with FcR block (BD Biosciences) and then stained with anti-lineage, anti-Sca-1, anti-c-kit, anti-CD150, and anti-CD48 antibodies to quantitate phenotypically defined HSPC populations. After staining, cells were fixed with 1% paraformaldehyde and analyzed by flow cytometry. For stromal cell analysis, BM cells were first stained with anti-CD45, anti-ter119, anti-CD51, anti-PDGFRa, anti-CD31 and anti-VE-cadherin antibodies followed by fixation and permeabilization, and then stained with anti-Nestin and anti-KAP1 antibodies. Total and mitochondrial ROS was measured using CellROX and MitoTracker probe, respectively, using flow cytometry.
HSPC and MSC co-culture and hematopoietic stem cell transplantation
To achieve ex vivo MSC expansion, CD45 and Ter119 depleted mouse BM cells were cultured in mesencult expansion medium for two-three weeks. For MSC/HSPC coculture, HSPC enriched lineage negative cells from young mice (CD45.2) were plated on ex vivo expanded MSC derived either from young or old WT mice or CXCR4 KO mice for 7 days in StemSpan™ medium. At the end of co-culture, CD45.2+ hematopoietic cells were purified from the MSC cultures by FACS sorting and 0.5 × 106 cells were competitively transplanted (1:1 ratio) into lethally irradiated (1100 cGy; split dose) young WT mice as described [12]. Donor chimerism in BM and tri-lineage reconstitution in peripheral blood was measured at 6months post transplantation.
CFU-F assay
Freshly harvested mouse BM cells were enriched for the CD45 and Ter119neg fraction using anti-CD45 and anti-ter119 magnetic beads, and 1×106 CD45 and Ter119 negative cells were cultured in 6-well tissue culture plates using MesenCult™ expansion media with 10% FBS. One half of the media was replaced after 7 days and on day 14 cells were stained with Giemsa staining solution (EMD Chemicals, Billerica, MA) and colonies enumerated.
ELISA
BM extracellular fluid (BMEF) was obtained by flushing one femur with 1 mL ice-cold PBS followed by centrifugation at 400 × g for 3 minutes. Cell free supernatants were used to measure SDF-1, SCF and VEGF by ELISA (R&D Systems). All samples were from individual mice and tested in duplicate.
Migration assay
Bone marrow CD45 and Ter119 negative cells were placed in upper chambers of transwell inserts (5 um) (Corning, Lowell, MA) and their migration to rm/rh SDF-1 (100 ng/ml; R&D system) was quantitated by flow cytometry after 4 hours as we described [12]. Percentage migration was calculated by dividing total cells migrated to the lower well by the cell input multiplied by 100. MSC cell migration was determined by comparison of the proportion of MSCs in input and migrated populations.
Statistical Analysis
All WT and KO mice were age and sex matched, and cages were randomly assigned to treatment groups. The number of animals used in the experiments was estimated to give sufficient power (>90%) based on the effect sizes observed in our preliminary data. Grubbs method to identify the outliers and no animals were excluded from the analysis. In vivo and ex vitro processing steps were not blinded. All the statistical analyses were performed using Excel (Microsoft Corp, Redmond, WA) or Prism-GraphPad software (GraphPad, San Diego, CA). Statistical significance for binary comparisons was assessed by 2-tailed Student’s t-test; data were normally distributed with sufficiently equivalent variances. For comparison of more than two groups, ANOVA with post-hoc test was used. All data are reported as Mean ± SEM.
RESULTS
Aging reduces BM MSC number and clonal expansion
MSC are a crucial component of the BM HSC niche, supporting HSC function by producing cytokines, including stromal derived factor-1 (SDF-1), stem cell factor (SCF), vascular endothelial growth factor (VEGF), and angiopoietin. Because MSC play an essential role in HSC maintenance and HSC function declines with aging, we evaluated the influence of aging on BM MSC. We first quantitated CD45−Ter119−CD31−PDGFR+CD51+ MSC by flow cytometry and functional clonal expansion ability by in vitro CFU-F formation in old (20–24 month) mice and compared these to young 2–3 month old mice. MSC number and clonal expansion potential were significantly lower in old mice (Figure 1a, b). We next measured stromal cell derived HSC supporting factors in the BM extracellular fluid of old and young mice. SDF-1, SCF and VEGF levels were significantly lower in old mice (Figure 1c). Consistent with previous reports [8], we observed higher numbers of HPC enriched LSK (Lineage−Sca-1+c-Kit+) and HSC enriched SLAM-LSK (Lineage−Sca-1+c-Kit+CD48−CD150+) cells in the BM of old mice (Figure 1d). However, while phenotypically defined HPC, ie LSK cells, were elevated in old mice, functional CFU-C colony formation was dramatically reduced (Figure 1e).
Figure 1: Aging reduces BM mesenchymal stromal cell number and their clonal expansion.
(a) CD45−Ter119−CD31−CD51+PDGFRα+ MSC in the BM of young and old mice. (b) Quantitation of BM CFU-F. (c) SDF-1, SCF and VEGF in BM extracellular fluid of young and old mice. (d-e) LSK, SLAM LSK and CFU-C in the BM of young and old mice (f) Total and mitochondrial ROS in BM MSC of young and old mice. (g) Expression of phospho-Kap1 and phosphor H2AX in the BM MSC. All data are X±SEM; N= 4 mice/group, each assayed individually; *=P<0.05.
Oxidative stress is a major contributor to cellular aging. Elevated levels of reactive oxygen species (ROS) are considered toxic to cells as it induces oxidative damage to multiple cellular macromolecules [13]. To determine whether oxidative stress contributes to attrition of the MSC pool in the BM of old mice, we measured cytoplasmic and mitochondrial ROS. Marrow MSC from old mice express significantly higher levels of cytoplasmic and mitochondrial ROS compared to MSC from young mice (Figure 1f). Because elevated ROS can induce DNA damage, we also measured phosphorylated KAP1, a marker of DNA damage [14]. MSC from old mice showed significantly higher levels of Kap-1 phosphorylation compared to MSC from young mice (Figure 1g). In addition, H2AX, another marker of DNA damage, was also higher in the BM MSC of old mice (Figure 1g). These data suggest that ROS-mediated DNA damage may contribute to aging-related reduction in marrow MSC number and proliferative capacity.
Old MSC are sufficient to induce an HSC aging phenotype
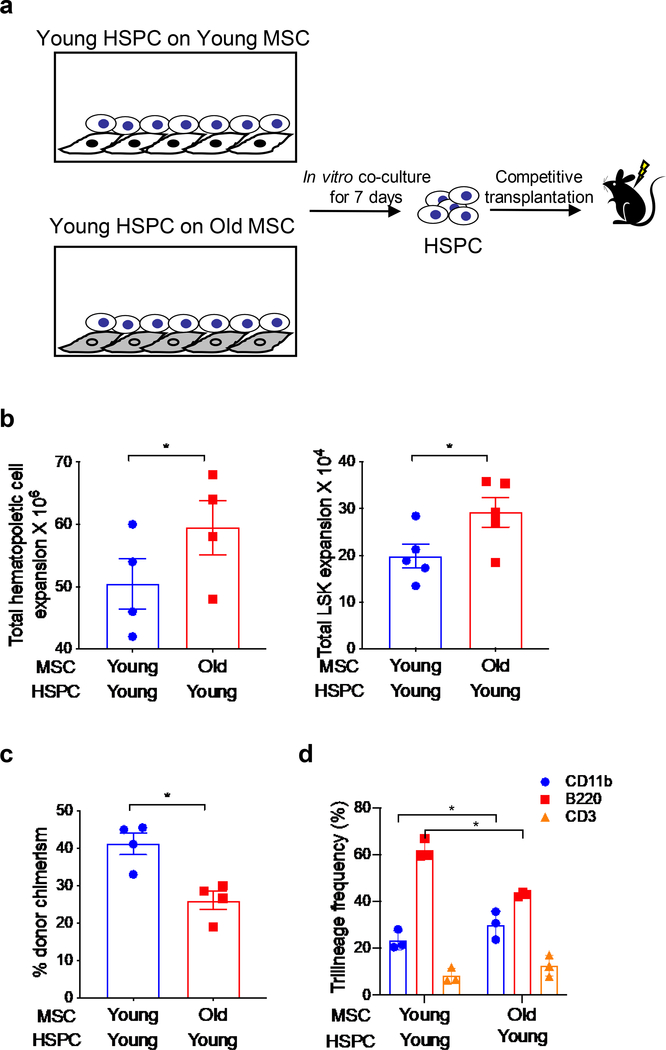
HSC aging is functionally defined by a decrease in repopulating activity and an increase in the differentiation/frequency of myeloid cells at the expense of lymphoid cells, i.e., myeloid skewing. To explore whether old MSC plays an instructive role in promoting aging-related hematopoietic phenotypes, we performed ex vivo co-culture of HSPC and MSC and quantitated the effect of MSC on HSPC number/expansion and HSC long-term repopulating activity (Figure 2a). When lineage negative BM cells from young mice were cultured with MSC from old mice an increase in total CD45.2+ hematopoietic cells and LSK cells was observed (Figure 2b). In contrast, young HSPC cultured with old MSC demonstrated a significant decrease in repopulating activity and contribution to peripheral blood chimerism compared with young HSPC cultured with young MSC when competitively transplanted at a 1:1 ratio in lethally irradiated young F1 hybrid recipient mice (CD45.1/CD45.2 (Figure 2c). In addition, recipients transplanted with young HSPC cultured on old MSC showed a significantly higher frequency of donor-derived CD11b+ myeloid cells and lower B220+ lymphoid cells, i.e., myeloid skew, compared to the young HSPC cultured on young MSC (Figure 2d). These data strongly suggest that old MSC, independent of other stromal constituents, can produce an aging phenotype in young HSPC.
Figure 2: Old MSCs can induce HSPCs aging phenotype.
(a) Schematic representation of culture system young HSPC with ex vivo expanded MSCs from young or old mice. (b) Quantitation of total hematopoietic cells and LSK expansion after culture of young HSPC with MSC from young or old mice for 7 days. Data are X±SEM; N= 5 independent cocultures; *=P<0.05. (c-d) Measurement of repopulation and trilineage differentiation capacity of HSPC co-cultured on young or old MSC in a competitive transplantation model. HSPC cultured on young or old MSC were mixed with competitor cells and transplanted into lethally irradiated young mice. At 6-month post transplantation, donor cell engraftment and myeloid and lymphoid frequency in the PB of recipient mice was measured by flow cytometry. Data are X±SEM; N= 4 mice/group, each assayed individually; *=P<0.05.
CXCR4 is reduced on old MSC and CXCR4 deficient MSC mimic an old MSC phenotype
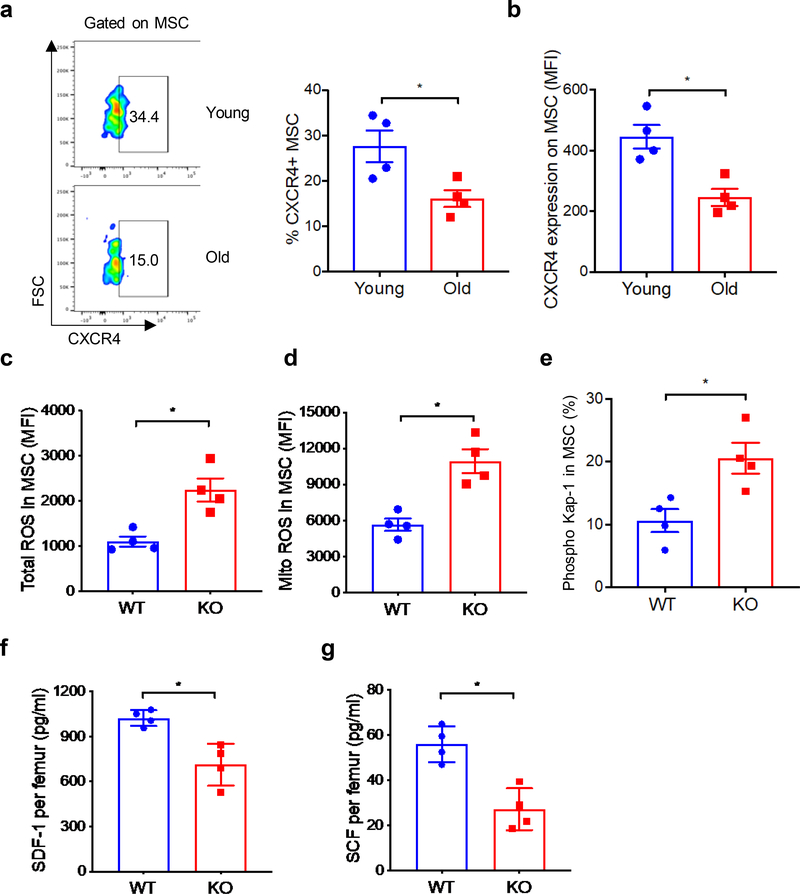
We recently found that CXCR4 gene deletion in BM stromal cells reduces hematopoietic regeneration after transplantation [12]. Because BM MSC express CXCR4, and aging impairs MSC activities, we tested whether alteration in CXCR4 expression contributes to aging-related MSC defects. We first measured CXCR4 expression on young versus old BM MSC. The percentage of MSC that express CXCR4 and mean fluorescent intensity (MFI) of CXCR4 was substantially reduced on MSC from old mice in comparison to MSC from young mice (Figure 3a, b).
Figure 3: CXCR4 expression is reduced on MSC from old mice and CXCR4 deficient MSC mimic an old MSC phenotype.
(a-b) CXCR4 expression on BM MSC (percentage positive and MFI) of young and old mice. (c-d) Total and mitochondrial ROS expression in MSC of WT and CXCR4 KO mice. (e) Phospho-Kap-1 expression in the MSC from WT and CXCR4 KO mice. (f-g) SDF-1 and SCF levels in the BM extracellular fluid of WT and CXCR4 KO mice. Data are X±SEM; N= 4 mice/group, each assayed individually; *=P<0.05.
Since MSC from old mice demonstrate higher levels of ROS than MSC from young mice, as described earlier, we tested whether CXCR4 gene deletion in MSC can enhance ROS production. At six-month post-CXCR4 gene deletion, similar to our observations with MSC from old mice, CXCR4-deficient MSC also showed higher expression of cellular and mitochondrial ROS (Figure 3c, d) compared to age-matched WT control mice. Consistent with MSC from old mice, CXCR4 deficient MSC also demonstrate enhanced DNA damage (Figure 3e). In addition, the HSC supporting factors SDF-1 and SCF were significantly lower in the BM milieu of CXCR4 deficient mice (Figure 3f, g). These data suggest that reduced CXCR4 expression on MSC may contribute to aging-mediated defect in MSC function.
CXCR4 deficiency in MSC induces an HSC aging phenotype
Since MSC from old mice express significantly less CXCR4 and CXCR4 deficient MSC functionally mimic old MSC, and our recent finding that CXCR4 deletion in BM stroma impairs hematopoietic regeneration [12], we tested the hypothesis of whether aging-related attenuation of CXCR4 expression/signaling in BM MSC contributes to an HSPC aging phenotype. We cultured HSPC from wild-type mice with ex vivo expanded MSC from wild-type mice or mice in which the CXCR4 gene was deleted using a tamoxifen-inducible Cre/loxP system and measured HSPC phenotype and repopulating ability after competitive transplantation (Figure 4a). Flow cytometric analysis validated that CXCR4 expression was essentially absent BM MSC of CXCR4 conditional KO mice after tamoxifen treatment (Figure 4b). Wild type HSPC cultured on CXCR4 deficient MSC demonstrated an old phenotype including higher LSK frequency (Figure 4c) and increased cell cycle (Figure 4d), compared to HSPC cultured on WT CXCR4 expressing MSC. Functionally, HSPC cultured on CXCR4 KO MSC demonstrated lower engraftment upon competitive transplant (Figure 4e) with myeloid skewed differentiation (Figure 4f). These data suggest that CXCR4 deficiency in MSC is sufficient to drive an aging HSPC phenotype.
Figure 4: CXCR4 deficiency in MSC induces HSPC aging phenotype.
(a) Schematic of WT HSPC co-cultured with ex vivo expanded MSC from WT or CXCR4 KO mice. (b) Measurement of CXCR4 expression on BM MSC of tamoxifen treated WT and CXCR4 conditional KO mice by flow cytometry. (c-d) LSK frequency and cycling after culturing the WT HSPC for 7 days on WT or CXCR4 KO MSC. (e-f) Measurement of hematopoietic reconstitution and myeloid differentiation capacity of WT HSPC cultured on WT or CXCR4 KO MSC after competitive transplantation. HSPC cultured on WT or CXCR4 KO MSC were mixed with competitor cells and transplanted into lethally irradiated young mice. At 6-month post transplantation, donor cell chimerism and myeloid cell and lymphoid frequency in the BM of recipient mice was measured by flow cytometry. Data are X±SEM; N=4–5 mice/group, each assayed individually; *=P<0.05.
The ROS scavenger NAC enhances CFU-F and reduces HSPC cycling in old or CXCR4 deficient MSC
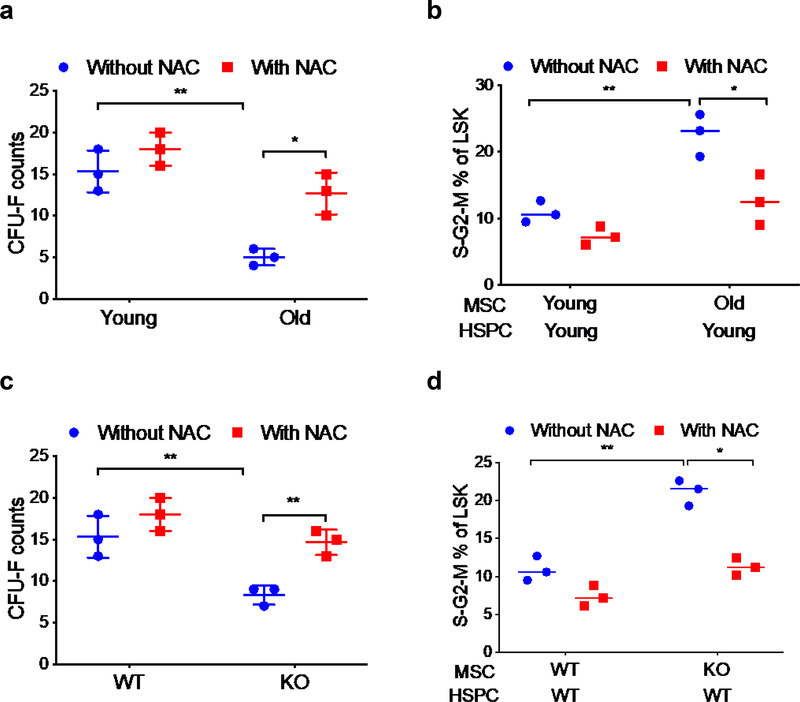
Because old and CXCR4 deficient MSC have higher levels of total and mitochondrial ROS, we hypothesized that reducing ROS levels in these MSC would improve their niche supporting activity and alleviate the HSPC aging phenotype. Consistent with previous results, MSC from old mice demonstrated significantly reduced CFU-F capacity (Figure 5a) and culture of MSC with the ROS scavenger N-acetyl-L-cysteine (NAC) for 7 days enhanced CFU-F formation by MSC from old mice, with little effect on CFU-F formation by young MSC (Figure 5a). In cultures of young HSPC with young or old MSC, inclusion of NAC in the cultures resulted in reduction of the elevated HSPC cycling rate normally seen with culture of young HSPC on old MSC to the levels normally observed with culture of young HSPC on young MSC (Figure 5b). Similarly, treatment of CXCR4 deficient MSC with NAC enhanced CFU-F formation and attenuated HSPC cycling (Figure 5c, d). These data demonstrate that treatment of old or CXCR4 deficient MSC with NAC to reduce ROS improves their clonal expansion and attenuates aging-related elevated HSPC cell cycle rate.
Figure 5: Treatment of old or CXCR4 deficient MSC with ROS scavenger enhances CFU-F and reduces HSPC cycling.
(a) CFU-F formation from young and old BM MSC cultured in the presence or absence of NAC. (b) Young HSPC cell cycle after culturing with young or old MSC for 7 days in the presence or absence of NAC. (c) CFU-F formation from WT or CXCR4 KO BM MSC cultured with or without NAC for 7 days. (d) Young HSPC cell cycle after culturing with WT or CXCR4 KO MSC for 7 days in the presence or absence of NAC. Data are X±SEM; N=3 experiments, each assayed individually; *=P<0.05.
DISCUSSION
Our studies suggest that age-associated reduction in CXCR4 expression on BM MSC impairs their HSC niche supporting activity resulting from increased ROS production, driving an HSC aging phenotype. We found that CXCR4 expression on BM MSC decreases with age and CXCR4 gene deletion accelerates the aged phenotype. Interestingly, old MSC, independent of other stromal constituents, can produce an aging phenotype in young HSPC. Mechanistically, CXCR4 signaling prevents BM MSC dysfunction by suppressing oxidative stress, as treatment of old or CXCR4 deficient MSC with NAC, improved their niche supporting activity, and attenuated the HSC aging phenotype.
Age-induced alterations in niche cell composition include decreased bone formation, increased adipogenesis, and changes in extracellular matrix components. It has been previously shown that the old BM microenvironment contributes to HSPC aging, as transplantation of young HSPC into old mice show reduced stem engraftment and myeloid skew [15]. In this context, our finding that co-culture of young HSPC exclusively with old MSC can induce an HSPC aging phenotype, suggests that old MSC, independent of other stromal constituents, can trigger HSPC aging. Given that MSC are the primary source of HSC maintenance factors, including SDF-1 and SCF-1 [16] and our finding of reduced levels of these factors in the BM of old mice, suggests that the deficit of these factors in the BM microenvironment is a major contributor to HSPC aging.
Reduced CXCR4 expression on BM Lineage−Sca-1+ progenitors likely contributes to impaired neovascularization in old mice [17]. Overexpression of CXCR4 in human MSC can reverse the age-related defect in their homing to BM [18]. Our finding that aging-related defects in HSPC function accompany the downregulation of CXCR4 expression on BM MSC, confirms a crucial role for MSC expressed CXCR4 in HSC niche regulation. This notion is further supported by the fact that the long-term (6-month) deletion of CXCR4 gene in young mice increases DNA damage in MSC, reduces their number and levels of HSC maintenance factors, and triggers HSPC aging phenotype after coculture of WT HSPC with CXCR4 deficient MSC. The CXCR4 deficiency mediated DNA damage in MSC seems to depend on the duration of CXCR4 gene deletion as, during the early stage of CXCR4 deletion (3-month post deletion), we recently observed similar levels of phospho-Kap-1 expression in the BM MSC of WT and CXCR4 KO mice [12].
Oxidative stress plays a critical role in the induction of aging-related DNA damage and senescence mediated primarily through the production of high levels of reactive oxygen species (ROS). Elevated ROS induces oxidative damage of several cellular macromolecules, including nucleic acids, leading to cellular senescence and aging. Antioxidants such as NAC can substantially revert the HSC aging. The CXCR4/SDF-1 axis has been shown to regulate the lifespan of HSC by limiting ROS generation and genotoxic stress in HSC [19], and interestingly, aging reduces CXCR4 expression on hematopoietic progenitor cells [17]. Consistent with HSPC findings, our data now show that aging related reduced expression of CXCR4 on MSC can increase ROS generation leading to defect in MSC function. Given that the aging-related reduced expression of CXCR4 contributes to oxidative stress in MSC and triggers the aging phenotype in HSPC, modulation of CXCR4 expression in MSC or supplementation of antioxidants may lead to novel interventions to alleviate the age-associated decline in immune/hematopoietic function.
ACKNOWLEDGEMENTS
This study is supported by US Public Health Service grants AG046246 (LMP, MAK and CMO), HL096305 (LMP), and a CCEH pilot grant (PS). Old mouse colony maintenance is supported by Department of the Army grants PR140433 (LP, CMO), PR140896 (CMO), and PR141527 (CMO). Flow cytometry was performed in the Flow Cytometry Resource Core Facility of the IU Simon Cancer Center (NCI P30 CA082709).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Eisenstaedt R, Penninx BW, & Woodman RC (2006). Anemia in the elderly: current understanding and emerging concepts. Blood Rev, 20(4), 213–226. [DOI] [PubMed] [Google Scholar]
- 2.Dorshkind K, & Swain S (2009). Age-associated declines in immune system development and function: causes, consequences, and reversal. Curr Opin Immunol, 21(4), 404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardner ID (1980). The effect of aging on susceptibility to infection. Rev Infect Dis, 2 (5), 801–810. [DOI] [PubMed] [Google Scholar]
- 4.Hassan M, & Abedi-Valugerdi M (2014). Hematologic malignancies in elderly patients. Haematologica, 99(7), 1124–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichtman MA, & Rowe JM (2004). The relationship of patient age to the pathobiology of the clonal myeloid diseases. Semin Oncol, 31(2), 185–197. [DOI] [PubMed] [Google Scholar]
- 6.Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, & Goodell MA (2007). Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol, 5(8), e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho RH, Sieburg HB, & Muller-Sieburg CE (2008). A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood, 111(12), 5553–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison SJ, Wandycz AM, Akashi K, Globerson A, & Weissman IL (1996). The aging of hematopoietic stem cells. Nat Med, 2(9), 1011–1016. [DOI] [PubMed] [Google Scholar]
- 9.Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, … Weissman IL (2011). Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A, 108(50), 20012–20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura Y, Arai F, Iwasaki H, Hosokawa K, Kobayashi I, Gomei Y, … Suda T (2010). Isolation and characterization of endosteal niche cell populations that regulate hematopoietic stem cells. Blood, 116(9), 1422–1432. [DOI] [PubMed] [Google Scholar]
- 11.Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y, & Frenette PS (2013). PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med, 210(7), 1351–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh P, Mohammad KS, & Pelus LM (2020). CXCR4 expression in the bone marrow microenvironment is required for hematopoietic stem and progenitor cell maintenance and early hematopoietic regeneration after myeloablation. Stem Cells. doi: 10.1002/stem.3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson AG, & Schadt EE (2014). The role of macromolecular damage in aging and age-related disease. J Gerontol A Biol Sci Med Sci, 69 Suppl 1, S28–32. [DOI] [PubMed] [Google Scholar]
- 14.White D, Rafalska-Metcalf IU, Ivanov AV, Corsinotti A, Peng H, Lee SC, … Rauscher F 3rd. (2012). The ATM substrate KAP1 controls DNA repair in heterochromatin: regulation by HP1 proteins and serine 473/824 phosphorylation. Mol Cancer Res, 10(3), 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ergen AV, Boles NC, & Goodell MA (2012). Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood, 119(11), 2500–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, … Frenette PS (2010). Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature, 466(7308), 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shao H, Xu Q, Wu Q, Ma Q, Salgueiro L, Wang J, … Yu H (2011). Defective CXCR4 expression in aged bone marrow cells impairs vascular regeneration. J Cell Mol Med, 15(10), 2046–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kyriakou C, Rabin N, Pizzey A, Nathwani A, & Yong K (2008). Factors that influence short-term homing of human bone marrow-derived mesenchymal stem cells in a xenogeneic animal model. Haematologica, 93(10), 1457–1465. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Depond M, He L, Foudi A, Kwarteng EO, Lauret E, … Wittner M (2016). CXCR4/CXCL12 axis counteracts hematopoietic stem cell exhaustion through selective protection against oxidative stress. Sci Rep, 6, 37827. [DOI] [PMC free article] [PubMed] [Google Scholar]