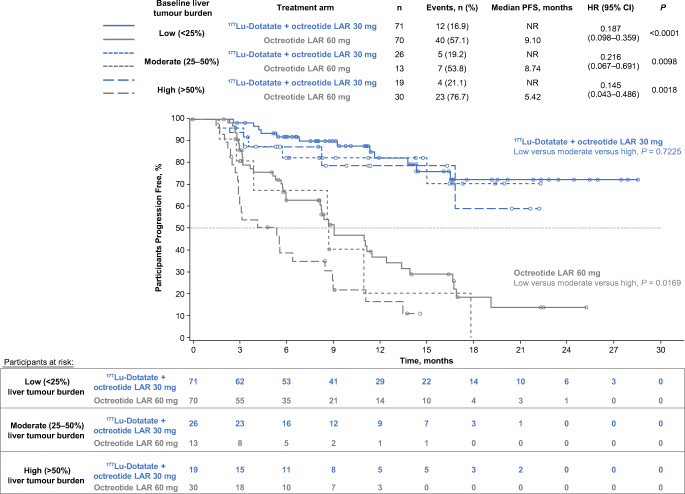
Fig. 1.
Kaplan-Meier analysis of progression-free survival by treatment arm (patients randomised to four cycles of peptide receptor radionuclide therapy with 177Lu-Dotatate + octreotide LAR 30 mg or octreotide LAR 60 mg) and baseline extent of liver tumour burden (low [< 25%], moderate [25–50%], or high [> 50%]). Liver tumour burden is calculated according to liver tumour volume divided by total liver volume by computed tomography or magnetic resonance imaging. Data cutoff: 24 July 2015. HRs with corresponding 95% CIs and P-values were estimated using a Cox regression model with randomised treatment, liver tumour burden at baseline, and liver tumour burden × randomised treatment interaction term as covariates. Log-rank test used for within-treatment arm comparisons of PFS. CI: confidence interval, HR: hazard ratio, LAR: long-acting release, NR: not reached, PFS: progression-free survival