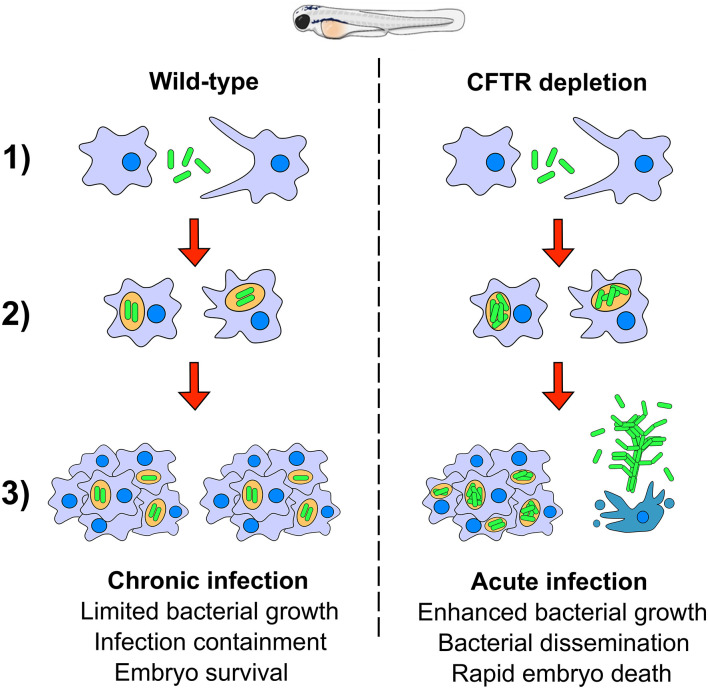
Figure 6.
A hypothetical schematic summarizing the pathogenesis of Mycobacterium fortuitum in both wild-type and CFTR backgrounds. (1) Following intravenous injection of M. fortuitum into zebrafish embryos, macrophages are rapidly recruited to the site of infection and phagocytose bacilli; (2) Once inside the phagosome, M. fortuitum is able to initially replicate, however in a wild-type scenario, host defenses are able to slow the bacterial expansion until further macrophages are recruited. Comparatively, following CFTR depletion, bacteria are able to rapidly expand within the phagosome, presumably due to defective host oxidative defense mechanisms. (3) Additional macrophages are recruited to the infection foci, forming the granuloma which acts to contain infection dissemination in wild-type embryos. In a proportion of wild-type embryos, we propose that macrophage apoptosis triggers the escape of bacilli to the extracellular space which facilitates the growth of bacilli into large corded aggregates, promoting zebrafish embryo death. In CFTR-depleted embryos, there are fewer granulomas and a greater proportion of cords which leads to more rapid embryonic death and a greater proportion of cords and abscesses.