Introduction
The unprecedented global pandemic caused by coronavirus 2 (SARS-Cov2) drew medical attention toward patients with co-morbid disorders, who are more exposed to prognostically unfavorable outcomes (1). A peculiar clinical scenario is represented by oncologic patients, who appear more susceptible to COVID-19 infection, as they may develop more severe symptoms than those observed in individuals with different comorbidity profiles (2).
Background and Rationale
Comorbid patients require multiple pharmacological therapies, which may, in turn, result in issues that Clinicians are asked to address quickly by considering the possible drug-drug interactions that may occur, with the aim of preventing reduced effectiveness or increased burden of adverse events (3). Generally speaking, the question of whether concomitant pharmacological therapies may threaten the safety of patients is usually answered in a context that acknowledges the treatment options for each single disease, allowing fair management of interactions on the basis of robust clinical evidence (4). On the other hand, in case of comorbidities occurring in COVID-19 patients, Physicians are now asked to answer the challenging question of whether interactions are possible between pharmacological treatments for COVID-19, which are not well-defined yet, and antineoplastic agents (5). In fact, whilst awaiting results from over 300 clinical trials currently underway, which aim to identify effective therapies against the COVID-19 syndrome, how drugs used for COVID-19 patients (e.g., hydroxychloroquine, antiviral agents, monoclonal antibodies) (6) may redundantly influence the pharmacokinetics and pharmacodynamics of cancer drugs (e.g., chemotherapy, hormonotherapy, targeted therapy, and immunotherapy) remains an object of investigation (7). This issue is crucial interest in patients bearing a cancer who are co-morbid for COVID-19, while remaining asymptomatic or paucisymptomatic. In these patients, a delay in the administration of a scheduled oncological treatment may have a meaningful impact on both survival and quality of life outcomes (8).
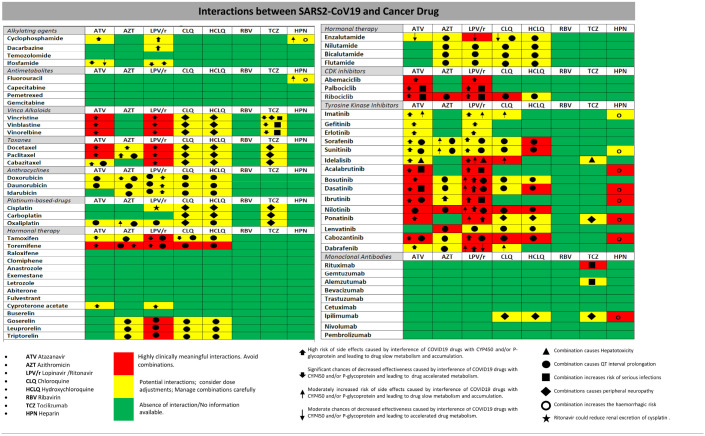
Attention should thus be focused on the interactions of the drugs most commonly used for COVID-19 with different classes of antineoplastic drugs (Table 1).
Table 1.
Checker illustrating likely interactions between antitumor agents and the drugs actually used in the SARS-Cov2 infection.
Interactions, which require major attention (red color), may occur between antiretroviral agents and chloroquine/hydroxychloroquine with Vinca alcaloids, Taxanes, Anti-estrogens, as well as CDK and TK inhibitors. Abbreviations and explanations in the lower part of the table. All checker's matches between drugs were verified in the current literature (9–11).
Discussion
In light of the array of possible interactions with systems of hepatic metabolism, such as cytochrome p450 (CYP450), as most of the actual antiviral agents used in COVID-19 infection are likely to impact on different CYP450 isozymes, dose adjustments of various drugs may be required for either cancer or SARS-Cov2 infection treatments. In fact, respective areas under the curve may be significantly different than expected in case of concomitant administration (12, 13). For example, either atazanavir or the combination lopinavir/ritonavir requires significant dose reduction of CDK4/6 inhibitors, and a full dose may be re-administered after 3–5 half-lives (14). With the same agents, administration of chloroquine should be intensely monitored because of the increased risk of QTc prolongation (15, 16). Also P-glycoprotein, a member of the ABC superfamily, regulating the efflux of drugs from cells, and similarly the multidrug and toxin extrusion protein-1 (MATE-1) are involved as targets for significant drug-drug interactions related to the administration of platinum compounds (17, 18). Thus, decreased efficacy and increased rates of resulting grade 3/4 adverse events (e.g., peripheral neuropathy, QT prolongation, increased risk of infection) need to be constantly and carefully addressed during concomitant anti-COVID-19-tumor treatments (19, 20). Thus, measurement of plasma/serum drug concentrations in the course of treatment appears an opportune procedure to avoid penalizing the efficacy of antiblastic treatments or exacerbation of toxicity. Interactions appear more likely to happen when using chemotherapics and/or hormonal therapy and may jeopardize the relatively satisfying results obtained, for example, in diseases such as the hormone dependent breast cancer or prostate cancer, which highly benefit of actually available treatments in terms of survival and quality of life.
In order to achieve a better knowledge on the management of different cancer treatment schedules in COVID-19 patients without compromising efficacy and safety, ad hoc observational clinical studies should be implemented with proper clinical endpoints pointing to the dose adjustments needed in case of emerging interactions.
Author Contributions
NS and RB: manuscript conception, writing, and revision. OB, CB, LS, and AM: manuscript elaboration and writing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunetti O, Derakhshani A, Baradaran B, Galvano A, Russo A, Silvestris N. COVID-19 infection in cancer patients: how can oncologists deal with these patients? Front Oncol. (2020) 10:734. 10.3389/fonc.2020.00734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nacher JC, Schwartz JM. A global view of drug-therapy interactions. BMC Pharmacol. (2008) 8:5. 10.1186/1471-2210-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palleria C, Di Paolo A, Giofrè C, Caglioti C, Leuzzi G, Siniscalchi A, et al. Pharmacokinetic drug-drug interaction and their implication in clinical management. J Res Med Sci. (2013) 18:601–10. [PMC free article] [PubMed] [Google Scholar]
- 5.Di Lorenzo G, Di Trolio R, Kozlakidis Z, Busto G, Ingenito C, Buonerba L, et al. COVID 19 therapies and anti-cancer drugs: a systematic review of recent literature. Crit Rev Oncol Hematol. (2020) 52:102991. 10.1016/j.critrevonc.2020.102991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA J Am Med Assoc. (2020) 323:1824–36. 10.1001/jama.2020.6019 [DOI] [PubMed] [Google Scholar]
- 7.Back D, Marzolini C, Hodge C, Marra F, Boyle A, Gibbons S, et al. COVID-19 treatment in patients with comorbidities: awareness of drug-drug interactions. Br J Clin Pharmacol. (2020). 10.1111/bcp.14358. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jafari A, Dadkhahfar S, Perseh S. Considerations for interactions of drugs used for the treatment of COVID-19 with anti-cancer treatments. Crit Rev Oncol Hematol. (2020) 151:102982. 10.1016/j.critrevonc.2020.102982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali W, Ahmed SBS, Abdalaziz AM, Elshafie MI, Mohammed HAA. Drug-drug interaction checker. J Clin Eng. (2019) 44:125–34. 10.1097/jce.0000000000000347 [DOI] [Google Scholar]
- 10.Marcath LA, Xi J, Hoylman EK, Kidwell KM, Kraft SL, Hertz DL. Comparison of nine tools for screening drug-drug interactions of oral oncolytics. J Oncol Pract. (2018) 14:e368–74. 10.1200/JOP.18.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Drug Interaction Information From Radboud UMC and The University of Liverpool. Available online at: https://cancer-druginteractions.org/ (accessed May 18, 2020).
- 12.Lynch T, Price A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Phys. (2007) 76:391–6. [PubMed] [Google Scholar]
- 13.Tseng A, Foisy M. Important drug-drug interactions in HIV-infected persons on antiretroviral therapy: an update on new interactions between HIV and Non-HIV drugs. Curr Infect Dis Rep. (2012) 14:67–82. 10.1007/s11908-011-0229-1 [DOI] [PubMed] [Google Scholar]
- 14.André F, Stemmer ST, Hortobagyi GN, Stegert M, Germa C, Arteaga CL, et al. Ribociclib + letrozole for first-line treatment of HR+, HER2– ABC: efficacy, safety, and pharmacokinetics. Eur J Cancer. (2016) 69:S1 10.1016/s0959-8049(16)32619-3 [DOI] [Google Scholar]
- 15.Thill M, Schmidt M. Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer. Therap Adv Med Oncol. (2018) 10:1758835918793326. 10.1177/1758835918793326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez-Chávez A, van Hoppe S, Rosing H, Lebre MC, Tibben M, Beijnen JH, et al. P-glycoprotein limits ribociclib brain exposure and CYP3A4 restricts its oral bioavailability. Mol Pharm. (2019) 16:3842–52. 10.1021/acs.molpharmaceut.9b00475 [DOI] [PubMed] [Google Scholar]
- 17.Harrach S, Ciarimboli G. Role of transporters in the distribution of platinum-based drugs. Front Pharmacol. (2015) 6:85. 10.3389/fphar.2015.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mealey KL, Fidel J. P-glycoprotein mediated drug interactions in animals and humans with cancer. J Veter Int Med. (2015) 29:1–6. 10.1111/jvim.12525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bossaer JB, Thomas CM. Drug interaction database sensitivity with oral antineoplastics: an exploratory analysis. J Oncol Pract. (2017) 13:e217–22. 10.1200/jop.2016.016212 [DOI] [PubMed] [Google Scholar]
- 20.Ford N, Vitoria M, Rangaraj A, Norris SL, Calmy A, Doherty M. Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS or COVID-19: initial assessment. J Int AIDS Soc. (2020) 23:e25489 10.1002/jia2.25489 [DOI] [PMC free article] [PubMed] [Google Scholar]