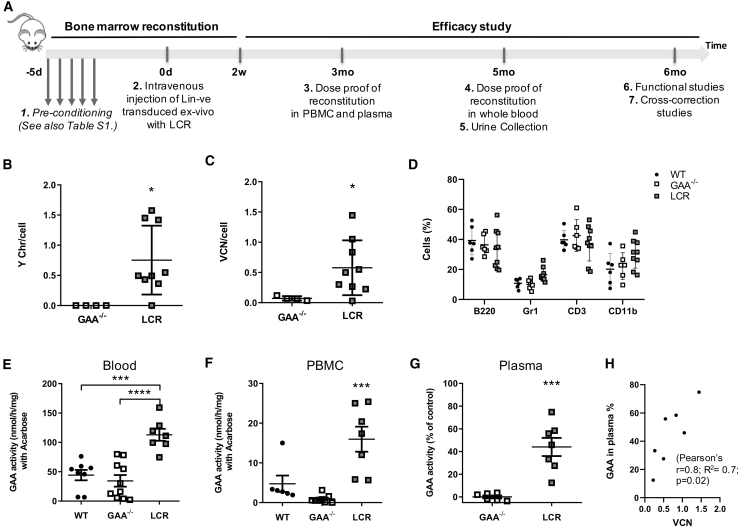
Figure 3.
LV.LCR-EFS.GAA HSC Gene Therapy in a Murine Model of Pompe Disease
Lin-ve cells from male GAA−/− mice were transduced ex vivo with LV.LCR-EFS.GAA (LCR) and transplanted into conditioned GAA−/− female mice. (A) Scheme of the experimental design, which illustrates the main steps and time points for the bone marrow reconstitution and efficacy study. (B and C) Engraftment (B) and VCN/cell (C) were measured in PBMCs at 12 weeks post-transplant. (D) Percentage of blood cells marked for B220, Gr1, CD3, or CD11b in control wild-type (●), control GAA−/− (empty square), and treated mice (gray-filled square) at 12 weeks after transplant. (E–G) The GAA expression was measured as GAA specific activity in (E) whole blood, (F) PBMCs, and (G) plasma, in which it is expressed as the percentage of increase compared to the GAA−/− control. (H) Pearson correlation analysis between GAA activity in plasma and VCN/cell detected in PBMCs of treated mice at 12 weeks after transplant (r = 0.8, R2 = 0.7, ∗p = 0.02). Data are shown as means ± SEM of three independent experiments for a total of n = 6–10 mice per group.