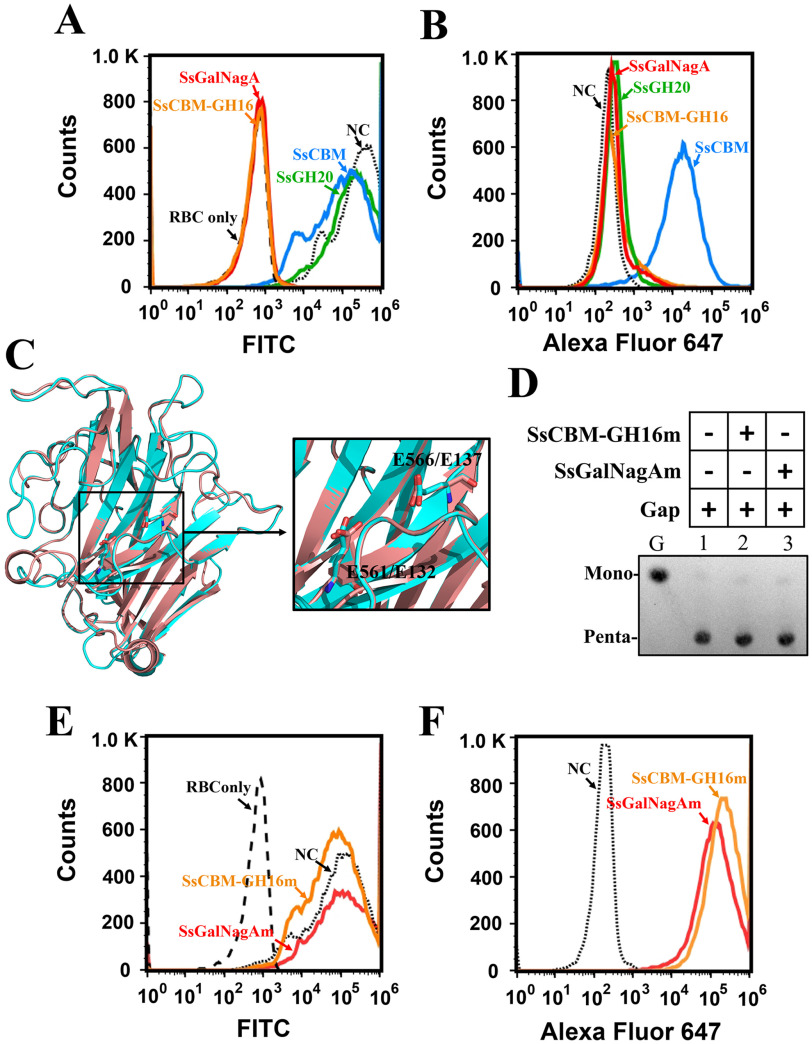
Figure 7.
The removal and binding of the terminal αGal epitope on swine erythrocytes by SsCBM, SsCBM-GH16, and full-length SsGalNagA. A, FCA detection of αGal epitope removal from swine erythrocytes. The erythrocyte solution (10%) was incubated with PBS–BSA (NC, negative control) or the indicated protein. The presence of the terminal αGal epitope was detected by FITC–BSI-B4 using FCA. The erythrocyte solution was detected and used as a control (Red blood cell only (RBC only)), B, FCA detection of protein binding to swine erythrocytes. The erythrocyte suspension (10%) was incubated with PBS–BSA (NC) or the indicated protein. The bound proteins were detected by Alexa Fluor 647 using FCA. C, superpositioning of structures of SsGH16 (cyan) and GH16 laminarinase from Thermotoga maritima MSB8 (pink; PDB entry 3b01). The structure of SsGH16 was modeled by SWISS-MODEL using 3b01 as the template. The catalytic residues, viz., E561 and E566 in SsGH16 and E132 and E137 in 3b01, are shown as stick models. The image on the right is an enlargement of the area framed on the left. D, TLC analysis of the hydrolysis of Gap by SsCBM-GH16m and SsGalNagAm. E, FCA detection of αGal epitope removal from swine erythrocytes by SsCBM-GH16m and SsGalNagAm. F, FCA detection of SsCBM-GH16m and SsGalNagAm binding to swine erythrocytes.