Figure 2:
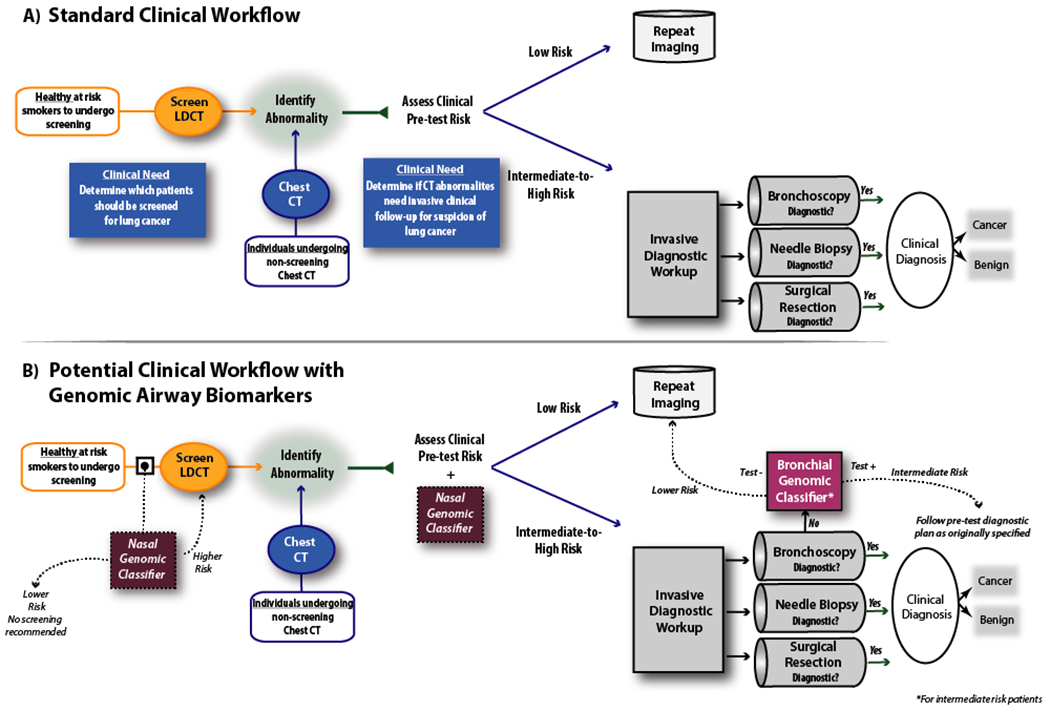
Potential clinical applications of airway molecular biomarkers for the early detection of lung cancer. A) In current practice, pulmonary nodules are discovered either due to lung cancer screening based on clinical risk factors, as findings incidental to other imaging studies, or during the work up of symptomatic patients. In the current diagnostic algorithm for indeterminate pulmonary nodules established by the American College of Chest Physicians (ACCP), based on the pretest probability of malignancy, patients determined to be low risk will typically undergo continued surveillance while higher risk patients will undergo invasive testing. However, there is a clinical unmet need to better stratify those subjects at intermediate pre-test risk into CT surveillance vs. invasive biopsy as well as an unmet need to identify which subjects should undergo CT screening. B) Molecular biomarkers have the potential to impact the current diagnostic workflow of patients with indeterminate pulmonary nodules. Current biomarkers measured in histologically normal-appearing bronchial airway tissue can serve to identify patients with non-diagnostic bronchoscopies who are at low risk for lung cancer and can be followed with continued imaging surveillance. Future biomarkers measured in histologically normal-appearing nasal airway tissue could help identify at-risk individuals for CT-based lung cancer screening or direct diagnostic workup in individuals with suspect lung nodules who are not otherwise undergoing bronchoscopy. CT = Computed Tomography. LDCT = Low Dose Computed Tomography.
