Table 3.
Substrate scope for the deaminative coupling of pyridinium salts with carboxylic acid derivatives.
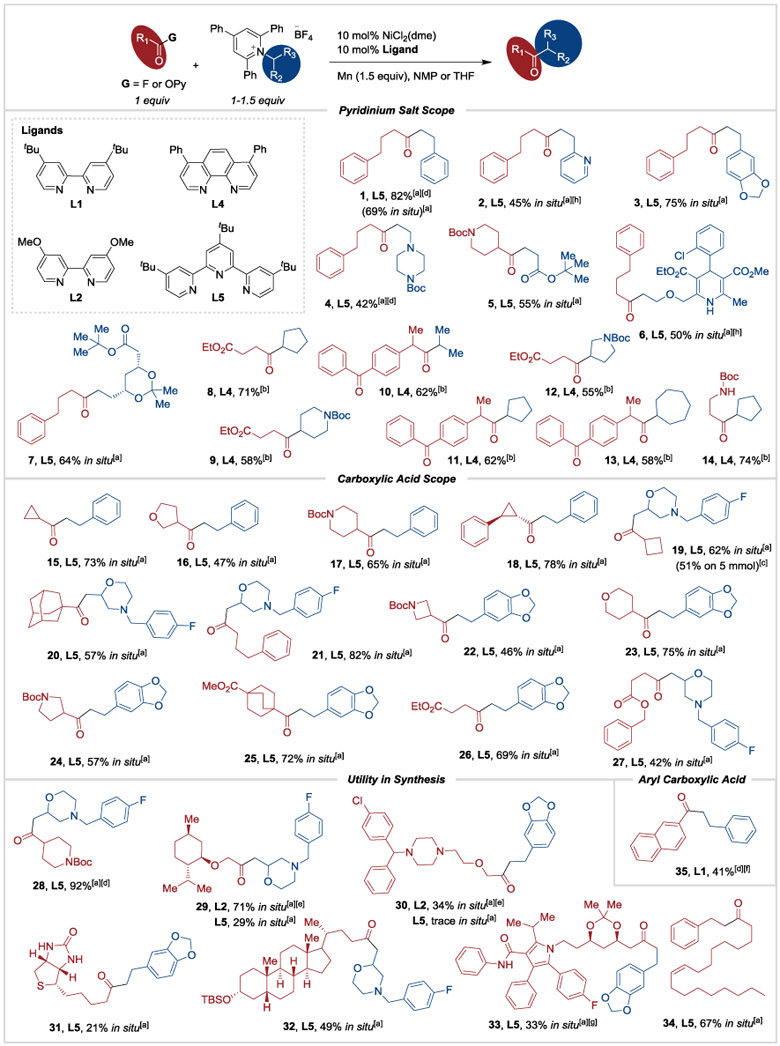 |
After initial acid fluoride formation using carboxylic acid (0.5 mmol, 1 equiv), TFFH (0.5 mmol, 1 equiv) and proton sponge (0.5 mmol, 1 equiv), the coupling was run on 0.5 mmol scale with a 1:1 ratio of starting materials, ligand L5 (0.05 mmol, 10 mol%), Mn (0.75 mmol, 1.5 equiv) in NMP (3 mL) at 60 °C for 24 h.
Reaction run at 0.5 mmol scale using pyridinium salt (0.75 mmol, 1.5 equiv) and pre-formed 2-pyridyl ester (0.5 mmol, 1 equiv) with NiBr2(dme) (0.05 mmol, 10 mol%), ligand L4 (0.05 mmol, 10 mol%), Mn (0.75 mmol, 1.5 equiv) in THF at r.t. for 24 h.
Reaction run on 5 mmol scale on benchtop, see Supporting Information.
Pre-formed acyl fluoride was used.
L2 used instead of L5.
L1 was used instead of L5.
Reaction run on 0.178 mmol scale at standard concentration.
NMR yield shown. Product was not separated from impurities, see Supporting Information for details.
Boc = tert-butoxycarbonyl, TBS = tert-butyldimethylsilyl.
