Figure 7. Depletion of PPME1, an Endogenous Inhibitory Protein of PP2A, Induces Prometaphase Cell Cycle Arrest in T-ALL Cells through Formation of the PP2A-B56ε Holoenzyme.
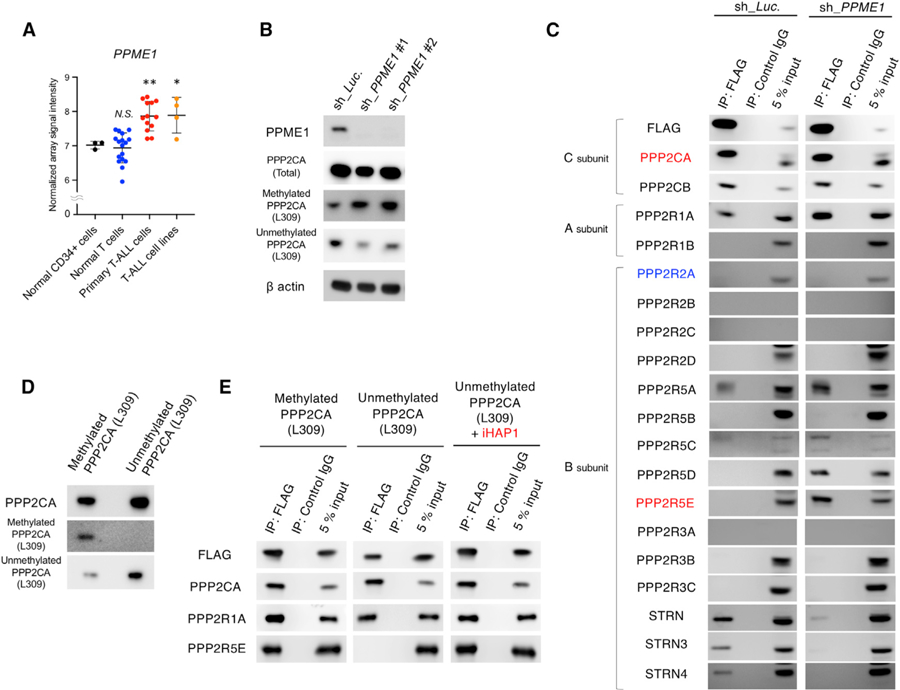
(A) The mRNA expression levels of PPME1 were compared in human normal CD34+ hematopoietic cells (n = 3), normal differentiated T cells (n = 17), patient-derived primary T-ALL cells (n = 13), and T-ALL cell lines (n = 4; Jurkat, KARPAS45, MOLT3, and MOLT16 [GSE48558]).
(B) Representative western blots of lysates of KOPT-K1 cells expressing doxycycline-induced shRNAs targeting PPME1 (sh_PPME1 #1 and #2) or the luciferase control (sh_Luc). The protein expression levels of PPME1, total PPP2CA, methylated PPP2CA at L309, unmethylated PPP2CA at L309, and b actin were examined by immunoblotting with specific antibodies.
(C) Coimmunoprecipitation assay using anti-FLAG tag antibody in cell lysate from KOPT-K1 cells expressing FLAG-tagged PPP2CA andPPP2CB and shRNAs targeting PPME1 (sh_PPME1 #1 and #2) or the luciferase control (sh_Luc.). Expression of shRNAs was induced with 3 µM doxycycline for 48 h. Immunoprecipitates were blotted with antibodies uniquely detecting each of the PP2A subunits.
(D) Representative western blot images of methylated and unmethylated PPP2CA proteins immunoprecipitated from transfected insect cells. Methylation of PPP2CA at L309 was carried out by incubation with LCMT1, demethylation of PPP2CA at L309 was carried out by incubation with PPME1, and products were detected by antibodies against methylated or unmethylated PPP2CA L309.
(E) Western blots with the indicated antibodies of coimmunoprecipitated proteins with anti-FLAG antibody of insect-produced subunit proteins, including methylated or unmethylated FLAG-tagged PPP2CA, PPP2R1A, and PPP2R5E (B56ε), after 1-h incubation with or without 1 µM iHAP1.
