Abstract
Background
Health-care workers are thought to be highly exposed to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. We aimed to investigate the prevalence of antibodies against SARS-CoV-2 in health-care workers and the proportion of seroconverted health-care workers with previous symptoms of COVID-19.
Methods
In this observational cohort study, screening was offered to health-care workers in the Capital Region of Denmark, including medical, nursing, and other students who were associated with hospitals in the region. Screening included point-of-care tests for IgM and IgG antibodies against SARS-CoV-2. Test results and participant characteristics were recorded. Results were compared with findings in blood donors in the Capital Region in the study period.
Findings
Between April 15 and April 23, 2020, we screened 29 295 health-care workers, of whom 28 792 (98·28%) provided their test results. We identified 1163 (4·04% [95% CI 3·82–4·27]) seropositive health-care workers. Seroprevalence was higher in health-care workers than in blood donors (142 [3·04%] of 4672; risk ratio [RR] 1·33 [95% CI 1·12–1·58]; p<0·001). Seroprevalence was higher in male health-care workers (331 [5·45%] of 6077) than in female health-care workers (832 [3·66%] of 22 715; RR 1·49 [1·31–1·68]; p<0·001). Frontline health-care workers working in hospitals had a significantly higher seroprevalence (779 [4·55%] of 16 356) than health-care workers in other settings (384 [3·29%] of 11 657; RR 1·38 [1·22–1·56]; p<0·001). Health-care workers working on dedicated COVID-19 wards (95 [7·19%] of 1321) had a significantly higher seroprevalence than other frontline health-care workers working in hospitals (696 [4·35%] of 15 983; RR 1·65 [1·34–2·03]; p<0·001). 622 [53·5%] of 1163 seropositive participants reported symptoms attributable to SARS-CoV-2. Loss of taste or smell was the symptom that was most strongly associated with seropositivity (377 [32·39%] of 1164 participants with this symptom were seropositive vs 786 [2·84%] of 27 628 without this symptom; RR 11·38 [10·22–12·68]). The study is registered at ClinicalTrials.gov, NCT04346186.
Interpretation
The prevalence of health-care workers with antibodies against SARS-CoV-2 was low but higher than in blood donors. The risk of SARS-CoV-2 infection in health-care workers was related to exposure to infected patients. More than half of seropositive health-care workers reported symptoms attributable to COVID-19.
Funding
Lundbeck Foundation.
Introduction
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has evolved into a pandemic with almost 14 million confirmed cases and almost 600 000 deaths.1 One key question is that of the risk of COVID-19 among health-care workers. 9% of all individuals who tested positive for SARS-CoV-2 by PCR in Italy and 26% in Spain, where transmission of SARS-CoV-2 has been intense, were health-care workers.2, 3, 4
Italy and Spain were among the first and most severely hit European countries by COVID-19 with no or limited time to prepare the health-care sector and take precautions to reduce the spread of the infection to health-care workers. The number of infected health-care workers might be lower in countries with more time for preparation, sufficient access to protective equipment, and less dramatic development of the epidemic—ie, in Denmark the accumulated mortality per million inhabitants is 75, compared with 452 in Italy.
In patients who survive COVID-19, a certain degree of immunity against SARS-CoV-2 is expected.5, 6 Unless an effective vaccine is developed in the near future the pandemic is likely to continue until herd immunity is reached or the disease has been eliminated. The exact proportion of the population that needs to develop immunity against SARS-CoV-2 to ensure herd immunity is unknown but is suggested to be between 60% and 80%.7, 8 Up to four-fifths of SARS-CoV-2-infected individuals are asymptomatic.9 Therefore, knowing the rates of seropositivity in the community is important.
Systematic screening for antibodies that are developed against SARS-CoV-2 (seroprevalence) is a crucial tool for surveillance of the pandemic and to predict when herd immunity might be reached. Health-care workers are expected to be one of the groups that are most exposed to SARS-CoV-2 infection, and surveillance of the proportion of seropositive health-care workers is an important indicator of the spread of SARS-CoV-2.
Research in context.
Evidence before this study
We searched PubMed for articles published from Jan 1 to May 5, 2020, for articles on screening and severe acute respiratory coronavirus 2 (SARS-CoV-2) in health-care workers. No language restrictions were applied. Search terms were (“SARS-CoV-2” OR “COVID-19”) AND (“healthcare personnel” OR “health*care workers” OR “healthcare workers” OR “doctors” OR “nurses”) AND (“screening” OR “test”). We identified three studies investigating the prevalence of SARS-CoV-2, which found that 11–18% of health-care workers were SARS-CoV-2-positive by PCR of pharyngeal swabs.
Added value of this study
This study is one of the first to screen for the seroprevalence of IgM and IgG antibodies against SARS-CoV-2. Furthermore, this is one of the largest screening studies done among health-care workers in relation to SARS-CoV-2. Health-care workers had an increased rate of seropositivity compared with Danish blood donors, who served as a proxy for the general population. Increased exposure to patients with COVID-19 was related to higher rates of seropositivity, as was increased patient contact in general. Younger health-care workers and men also had significantly higher levels of seropositivity than older health-care workers and women. The symptom that was most related to prevalence of seroconversion was loss of smell or taste. Just over half of seropositive participants reported symptoms that were attributable to COVID-19 and a fifth reported no symptoms at all.
Implications of all the available evidence
COVID-19 could be a work-related illness in health-care workers. In many European countries, men have been reported to be over-represented among COVID-19 cases. Our results cannot explain the high number of male fatalities but a higher rate of transmission in men is likely to be part of the explanation. Previously, loss of taste and smell has been found to be related to COVID-19, which was reiterated in this study. Our findings emphasise that the increased concern and risk of SARS-CoV-2 among health-care workers should be addressed in health-care policy making.
We aimed to investigate the seroprevalence in health-care workers, compared with that of the general population, as well as between subgroups of health-care workers.
Methods
Study design and participants
In this observational cohort study, a screening group consisting of senior consultants and professors, who were employed in the Capital Region of Denmark, initiated, led, organised, and ran a screening programme for antibodies against SARS-CoV-2.
All somatic, psychiatric, prehospital staff, and staff at specialised health-care institutions employed in the Capital Region of Denmark (1·84 million inhabitants) who considered themselves to have any contact with patients in relation to their work were invited to participate in this study. Voluntary screening was supported by the administrative and political systems in the region. Invitations for screening were posted on all internal hospital websites and sent out individually to all employees through the Danish governmental, personal, password-protected, email system (e-Boks). Medical students, nursing students, and all other students associated with the hospitals in the region were also invited to participate. Screening took place April 15–17, and April 20–22, 2020. Frontline in-hospital health-care workers were defined as doctors, nurses, assistant nurses, and medical and nursing students.
This study was registered with the Danish Data Protection Authorities (P-2020-361). This study was presented to the regional scientific ethics committee of the Capital Region, who concluded that the study did not require a scientific ethical approval (Jnr-H-20026288).
Procedures
Each clinical department was asked to establish a dedicated station where staff could have a blood sample taken at their own department for the lateral flow assay for IgM and IgG. A contact person was appointed at each department for local organisation. Sampling tubes, lateral flow assays, syringes, disinfection swabs and tourniquets, etc, were delivered by the screening group. Additionally, a temporary blood sampling clinic was established at each hospital.
SARS-CoV-2 IgG and IgM antibodies were tested in whole blood by a point-of-care test according to the manufacturer's recommendations (Livzon Diagnostics, Zhuhai, Guangdong, China). From a butterfly needle, one drop of blood followed by two drops of buffer (isotonic saline) were added to the test chamber in each of the two tests (IgM and IgG). The test results were read after 15 min. When no control line appeared or if the reading chamber was discoloured by blood the test was repeated. Test results were binary and read by the individual participant, assisted by local staff if needed. Instructions on how to read the results were posted online, in a video instruction, and sent by email to all participants. Inconclusive test results were treated as negative unless otherwise stated. Participants were categorised as seropositive if they had developed IgM or IgG, or both.
The manufacturer reported a sensitivity of 90·6% and a specificity of 99·2% for the point-of-care test combined. We internally validated the test, using 651 plasma samples from blood donors giving blood in the winter seasons before November, 2019 (three [<1%] reactive of 651 samples, one [<1%] inconclusive). Specificity was estimated to be 99·5% [95% CI 98·7–99·9]. Samples from 155 patients with previous documented SARS-CoV-2 (confirmed by PCR) were tested; 128 were reactive. Sensitivity was thus estimated to be 82·5% (75·3–88·4; appendix pp 18–22).
Participants were also asked to complete a survey. Participants accessed the survey through a link sent to their e-Boks or via a QR code at the blood sampling clinics. Participants completed the survey using a smartphone or computer. In the survey, participants were asked to give information about demographics, type of work, history of contact with patients with diagnosed COVID-19, and symptoms of infection (appendix pp 23, 24). Study data were collected and managed using Research Electronic Data Capture, a web-based, electronic data capture tool that is secure, hosted at the Capital Regions server.10, 11 Participation in the survey was voluntary and antibody screening was also provided to staff who did not wish to participate in the survey.
For comparative purposes, SARS-CoV-2 screening results from blood donors in the same period and same region were anonymously extracted from the Danish blood bank production system. Blood donors were screened with the same Livzon point-of-care test. This group was used as a proxy for the general population, aged 18–64 (the age range of blood donors).
Statistical analysis
The primary outcome was the proportion of the study population with a positive antibody test for SARS-CoV-2. Calculations were done using R (version 6.3.1; appendix pp 6–11. All results were presented as crude percentages. Possible associations between exposures and the primary outcome were explored by risk ratios (RRs). RRs were calculated as the probability of the outcome in the exposed group compared with that in the unexposed group. RRs were presented with 95% CIs, calculated using the normal approximation (Wald) and the significance (Fisher's exact test) as implemented in the R package epitools. A p value of less than 0·05 was considered significant. As a sensitivity analysis we adjusted the apparent (crude) prevalence in our cohort for the sensitivity and specificity of the point-of-care test to estimate the true prevalence in our cohort. We used the method suggested by Rogan and Gladen12 to calculate the true prevalence and 95% CI adjusted for the sensitivity and specificity of a test. The adjusted calculations were done using the epiR package (appendix pp 10, 11). To estimate the prevalence of patients with SARS-CoV-2 antibodies without symptoms of COVID-19, we calculated the probability of having symptoms of COVID-19, adjusted for the probability of seronegative participants having symptoms (appendix pp 10, 11, 15, 16). This study is registered at CinicalTrials.gov, NCT04346186.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between April 15 and April 23, 2020, 29 884 health-care workers were invited to take part in the study. 29 117 (97%) participants were included, of which 28 792 (98%) provided SARS-CoV-2 antibody results (appendix p 44). The mean age was 44·4 years (SD 12·6) years. 22 715 (78·9%) participants were female and 6077 (21·1%) were male (table 1 ; appendix p 25). Baseline characteristics for participants who completed the questionnaire from home (n=227; 0·78%) and could not do the point-of-care test or did not provide the point-of-care test results (n=98; 0·33%) are given in the appendix (p 26).
Table 1.
Baseline characteristics
| Seronegative (n=27 629) | IgG or IgM positive (n=1163) | ||
|---|---|---|---|
| Age, years | 44·5 (12·5) | 43·3 (13·5) | |
| Sex | |||
| Female | 21 883 (79·2%) | 832 (71·5%) | |
| Male | 5746 (20·8%) | 331 (28·5%) | |
| Body-mass index | 25·06 (4·69) | 24·83 (4·17) | |
| Any symptom of COVID-19 | 14 587 (52·8%) | 908 (78·1%) | |
| Diagnosed COVID-19 | 129 (0·5%) | 231 (19·9%) | |
Data are n (%) or mean (SD).
1163 had positive IgM or IgG, or both, antibodies corresponding to a seroprevalence of 4·04% (95% CI 3·82–4·27). Of these, 808 (2·81% [2·62–3·00]) had developed IgM and 768 (2·67% [2·49–2·86]) had developed IgG antibodies. 413 (1·43%) seropositive health-care workers had developed both IgG and IgM antibodies (1·43% [1·30–1·58]; appendix p 45).
4672 blood donors were tested during the same period as that of the screening of health-care workers. In the group of blood donors, 2502 (53·55%) were female, 2150 (46·45%) were male, and the mean age was 40·7 (SD 13·4 years. In this group the seroprevalence was 3·04% (95% CI 2·58–3·57; n=142) for IgG or IgM, 1·84% (1·49–2·27; n=86) for IgM only, and 1·97% (1·61–2·41; n=92) for IgG only. The seroprevalence was significantly higher in health-care worker compared with the blood donors (RR 1·33 [95% CI 1·12–1·58]; p=0·0008).
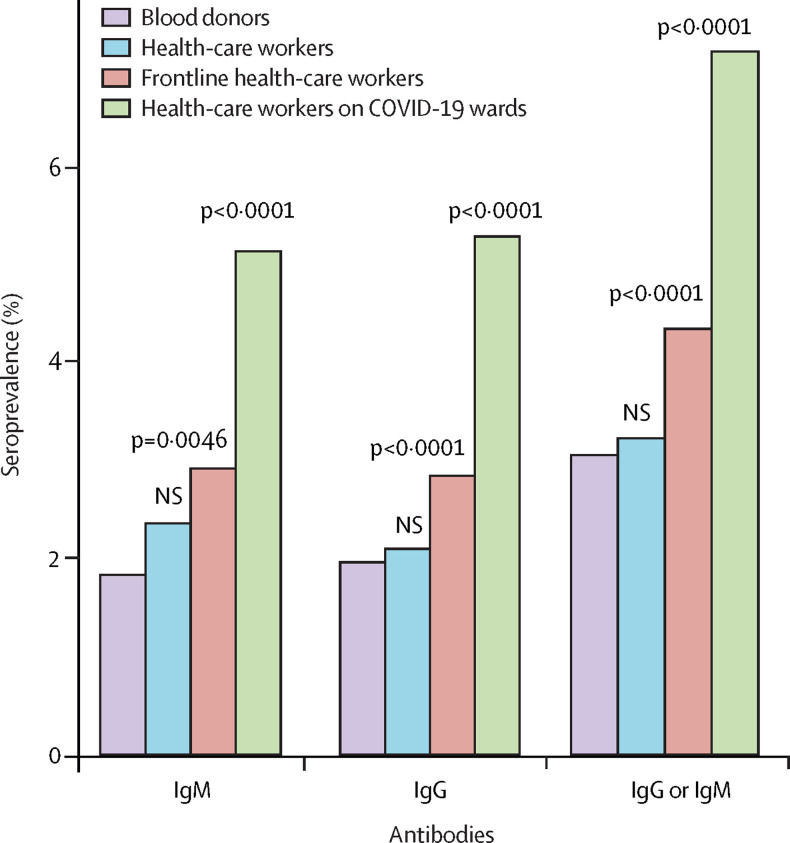
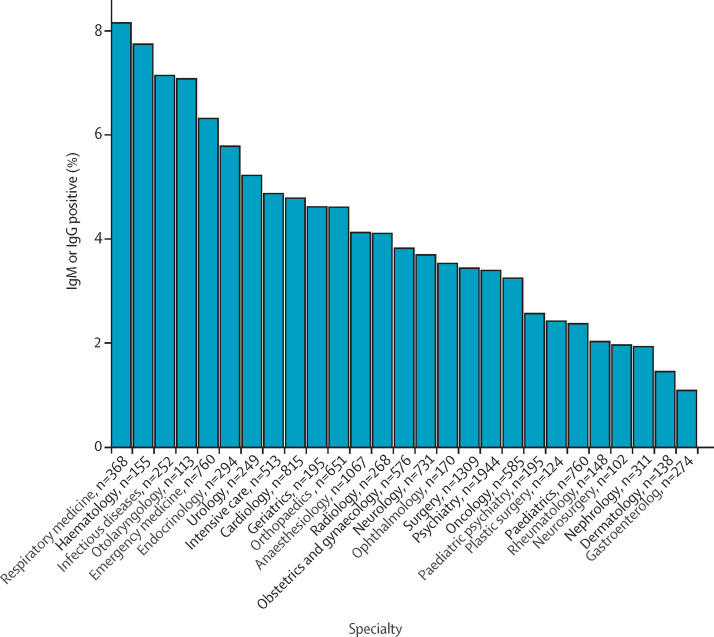
Table 2 shows the frequency of seropositivity for different job categories. The highest seroprevalence was seen in medical students and the lowest seroprevalence was observed among laboratory personnel (table 2). Frontline health-care workers working in hospitals (n=17 135 [4·55%]) had a significantly higher seroprevalence compared with the remaining health-care workers (n=11 657 [3·29%]; RR 1·38 [95% CI 1·22–1·56]; p<0·001; appendix p 27). 1321 (4·59%) participants reported working at a dedicated COVID-19 ward. We found a significantly increased seroprevalence in this group (n=95 [7·19%]) compared with other frontline in-hospital health-care workers (696 [4·35%] of 15 983; RR 1·65 [95% CI 1·34–2·03]; p<0·001; appendix p 28). Seroprevalences in blood donors, health-care worker, frontline health-care workers, and health-care workers working at dedicated COVID-19 wards are presented in figure 1 . Figure 2 shows the seroprevalence among doctors, nurses, and assisting nurses stratified by specialty (appendix p 29). Seroprevalences stratified according to hospital, somatic versus psychiatric hospitals, exposure to patients and other people, and regular versus no patient contact are given in the appendix (pp 31–34). We found a small but significant increase in the seroprevalence among health-care workers who reported regular patient contact (n=18 484) compared to those who reported no patient contact (n=4363) (4·26% vs 3·48%; RR 1·22 [95% CI 1·03–1·45]; p=0·02). This finding was only significant when considering both IgM and IgG antibodies. When examining whether seropositivity among health-care workers was correlated with the percentage of SARS-CoV-2-positive patients admitted at individual hospitals we found a significant Pearson's correlation coefficient of 0·85 (95% CI 0·13–0·98; p=0·03; appendix pp 17, 35, 46).
Table 2.
Frequencies of positive antibody tests stratified according to categories of professions
| Participants | IgM | IgG | IgM and IgG | IgM or IgG | |
|---|---|---|---|---|---|
| Physicians | 4698 | 137 (2·92%) | 112 (2·38%) | 58 (1·23%) | 191 (4·07%) |
| Nurses | 9963 | 283 (2·84%) | 265 (2·66%) | 146 (1·47%) | 402 (4·03%) |
| Assisting nurses | 1786 | 66 (3·70%) | 45 (2·52%) | 28 (1·57%) | 83 (4·65%) |
| Midwives | 501 | 9 (1·80%) | 6 (1·20%) | 4 (0·80%) | 11 (2·20%) |
| Radiographers | 342 | 9 (2·63%) | 9 (2·63%) | 6 (1·75%) | 12 (3·51%) |
| Laboratory personnel | 1292 | 18 (1·39%) | 15 (1·16%) | 8 (0·62%) | 25 (1·93%) |
| Medical students | 688 | 41 (5·96%) | 94 (13·66%) | 32 (4·65%) | 103 (14·97%) |
| Paramedics | 323 | 7 (2·17%) | 12 (3·72%) | 3 (0·93%) | 16 (4·95%) |
| Administrative staff | 2631 | 51 (1·94%) | 47 (1·79%) | 27 (1·03%) | 71 (2·70%) |
| Other | 6568 | 187 (2·79%) | 163 (2·72%) | 101 (1·40%) | 249 (4·11%) |
| All | 28 792 | 808 (2·81%) | 768 (2·67%) | 413 (1·43%) | 1163 (4·04%) |
Data are n or n (%).
Figure 1.
Seroprevalence according to job assignment compared with blood donors
Purple indicates blood donors serving as a proxy for the general population (n=4672). Blue indicates health-care workers not working on dedicated COVID-19 wards or frontline (n=11 488). Red indicates frontline health-care workers not working on dedicated COVID-19 wards (n=15 983). Green indicates health-care workers working on dedicated COVID-19 wards (n=1321). NS=not significant.
Figure 2.
Seroprevalence stratified according to specialty for doctors, nurses, and assisting nurses
Figure shows specialties with at least 100 participants.
Seroprevalence was significantly higher in male (n=331 [5·45%]) participants compared with female (n=832 [3·66%]) participants (RR 1·49 [95% CI 1·31–1·68]; p<0·001). Seropositivity stratified by sex for each of the antibodies are presented in the appendix (pp 36, 48). We found an association between age and seroprevalence, with participants younger than 30 years (n=4760 [5·29%]) having the highest seroprevalence compared with participants aged 30 years or older (n=24 032 [3·79%]; RR 1·40 [95% CI 1·22–1·60]; p<0·001; appendix p 48).
Seroprevalence stratified according to the presence of other diseases, smoking, and alcohol consumption are presented in the appendix (p 37). No major differences were found between the overall frequencies or between groups, except an increased seroprevalence in health-care workers with kidney disease; however, the sample size (n=69) was small.
908 (78·1%) of 1163 seropositive participants and 14 587 (52·8%) of 27 629 seronegative participants reported at least one COVID-19-associated symptom within 6 weeks before testing. From this finding and the assumption that the rate of COVID-19-like symptoms that were not attributable to COVID-19 were the same for COVID-19 patients, we estimated the proportion of patients with SARS-CoV-2 infection with no symptoms caused by the infection to be 541 (46·5%) of 1163 (appendix pp 15–16).
Having any symptom of COVID-19 (n=15 495) was associated with a significantly increased seroprevalence of IgG or IgM antibodies compared with asymptomatic health-care workers (908 [5·86%] of 15 496 vs 255 [1·92%] of 13 297; RR 3·05 [95% CI 2·66–3·50]; p<0·001). Loss of taste or smell (n=1164) was the symptom that was most strongly associated with being seropositive (32·39% vs 2·84%; RR 11·38 [10·22–12·68]). Fever (n=3203) was also strongly associated with seropositivity (15·0% vs 2·67%; RR 5·61 [5·03–6·27]). Participants reporting at least three symptoms (n=9797 [34%]) also had a significantly increased risk of being seropositive (7·41% vs 2·23%; RR 3·24 [2·88–3·64]). Table 3 shows a comparison of the seroprevalence of participants with any symptom, fever, loss of smell or taste, or at least three symptoms during the 6-week period (appendix pp 38–42). Results of the development of antibodies according to symptom onset are given in the appendix (p 49); over time, a decline in IgM seroconversion and a rise in IgG seroconversion were noted. Of the participants who had symptoms, 9517 (61·79%) reported being on sick leave due to symptoms. Staying at home with symptoms was strongly associated with seropositivity (RR 2·96 [95% CI 2·58–3·38]; p<0·0001).
Table 3.
Frequencies of positive antibody tests stratified according to symptoms of COVID-19
| IgM or IgG antibodies | No antibodies | p value | |
|---|---|---|---|
| Any symptom | 908 (5·86%) | 14 587 (94·14%) | <0·0001 |
| Fever | 480 (14·99%) | 2723 (85·01%) | <0·0001 |
| Loss of smell or taste | 377 (32·39%) | 787 (67·61%) | <0·0001 |
| ≥3 symptoms | 727 (7·39%) | 9113 (92·61%) | <0·0001 |
Data are n (%), unless otherwise indicated. p values were calculated using Fisher's exact test, comparing seropositivity with the factors listed.
4260 (14·5%) participants reported to have had a pharyngeal swab test because of suspicion of COVID-19. The results of antibody tests stratified by self-reported result of previous PCR by pharyngeal swab tests are given in the appendix (p 43). The prevalence of IgG and IgM antibodies were high among patients who had received a swab test regardless of the results of the swab. In patients with a PCR-positive SARS-CoV-2 test, 231 (64·17%) of 360 had a positive test for IgG or IgM antibodies. Details of health-care workers who were previously positive for SARS-CoV-2 by PCR, differentiated by time from specimen extracted to antibody testing, are given in the appendix (p 50).
We adjusted for the test characteristics of the point-of-care test results. The seroprevalence was estimated to be 2·86% (95% CI 2·63–3·10) for IgM antibodies, 2·69% (2·47–2·92) for IgG, and 4·36% (4·09–4·65) for IgG or IgM. To adjust for possible selection bias, we added the participants who were at home because of illness or reported no serology but reported that they had tested positive for COVID-19 on pharyngeal swab (n=73) to our adjusted estimates on seropositivity. This addition resulted in a seroprevalence of 3·13% (2·89–3·37) for IgM antibodies, 2·96% (2·73–3·20) for IgG, and 4·61% (4·34–4·90) for IgG or IgM.
Discussion
We found that the prevalence of seropositivity was significantly higher in health-care workers (although the difference was numerically small) than in blood donors who served as a proxy for the general population. The seroprevalence was significantly higher in frontline health-care workers working in hospitals compared with in other health-care workers, and health-care worker workings on dedicated COVID-19 wards had a significantly higher seroprevalence compared with other frontline health-care workers. We also found that the seroprevalence was significantly higher in men than in women, just over half of seropositive participants reported symptoms that were attributable to COVID-19, and a fifth reported no symptoms at all. Moreover, loss of taste or smell was the symptom most strongly associated with seropositivity.
In the COVID-19 pandemic, the provision of adequate health care to patients is fundamental to keep mortality low. The provision of state-of-the-art health care is highly reliant on professional staff that feel safe and well protected. Further, the importance of knowledge on susceptibility for transmission has been highlighted by WHO and in another study.13, 14 The first case of transmission in a Danish resident was discovered on Feb 27, 2020.15 2 weeks later the Danish authorities had closed large parts of society as part of a mitigation strategy because containment had failed in Denmark (appendix p 12). Testing in this study commenced in the 7th week after the first case of SARS-CoV-2 was detected in Denmark.
To our knowledge no other studies have offered systematic screening for antibodies against SARS-CoV-2 in health-care workers and no prior screening has been done in a population of this size. PCR-based identification of viral RNA in pharyngeal swabs has been used for screening in other cohorts.16, 17, 18 Previous studies (n=803, n=1533, and n=1654) on screening of symptomatic health-care workers found 11–18% to be SARS-CoV-2 positive.16, 17, 18 A screening study19 of asymptomatic health-care workers (n=1032) found 3% to be positive by PCR of pharyngeal swab specimen. One study18 speculated that frontline health-care workers would be more prone to viral transmission, but the study neither had enough power nor appropriate information on work assignment to answer this question. Health-care workers are not only expected to be exposed to SARS-CoV-2-infected patients in dedicated COVID-19 facilities but also patients with SARS-CoV-2 infection who are admitted for other reasons. Thus, a study screening for SARS-CoV-2 in 215 women admitted to give birth showed 33 (15%) to be positive and another screening of 80 residents in aged-care facilities showed 3 (4%) to be positive.20, 21 Our study clearly showed an increased risk of SARS-CoV-2 infection in health-care workers that was related to exposure to infected patients.
Immunity against COVID-19 was explored in a Chinese study in which all patients with COVID-19 had developed IgG antibodies against SARS-CoV-2 within 19 days after symptom onset.5 A study6 of 600 SARS-CoV-2-positive patients verified by PCR found 99% of the patients to have seroconverted within 50 days. Immunological status will be an important factor in risk stratification of exposed individuals and individuals at high risk of morbidity and mortality following COVID-19. In this study participants were categorised as seropositive if they had developed IgM or IgG antibodies. Because specificity is high for IgG and IgM combined but sensitivity is moderate, we found the combination to reflect the actual seroconversion rate best. Also, because testing was done at an early stage of the epidemic in Denmark, many participants who are IgM positive might develop IgG antibodies later. A complete seroconversion requires IgG antibodies against the virus, which we also reported.
The study of 215 pregnant women screened by PCR found 29 (88%) of 33 SARS-CoV-2-positive women were asymptomatic.20 Another study of mostly male homeless people found 129 (88%) of 147 people who tested positive by PCR to be asymptomatic.22 By contrast with these studies, we found that more than half of seropositive health-care workers reported symptoms attributable to COVID-19.
Among the different job categories, seroprevalence was highest among medical students. In the early days of transmission in Denmark a hotspot was found at a social gathering at a Copenhagen University club for medical students, which might explain the observed high rate of seroconversion in medical students.23 According to the European Centre for Disease Prevention and Control, critical illness due to COVID-19 is seen more frequently in men compared with women by a ratio of 2·7.24 In Denmark, 57% of SARS-CoV-2-positive individuals are female.25 We found that seroconversion was significantly more frequent in male health-care workers compared with female. This finding might indicate that the higher frequency of critical illness due to COVID-19 in men might to some extent reflect a higher proportion of infected men, and not only a higher susceptibility to SARS-CoV-2 in men. The report on surveillance for SARS-COV-2 does not include information on percentages of total sex-specific testing done. The sex-related difference in seroprevalence might be due to unknown underlying patterns of transmission or to different behaviour—eg, women might follow recommendations more carefully. The difference might also be of a biological origin if differences in immunological response or severity of disease between sexes exist. More research is needed to answer these questions.
Strengths of this study include the largest scope of screening against previous SARS-CoV-2 infection to date. The study had high participation and a high percentage of health-care workers submitted the questionnaire, which was web-based and easily accessible. By contrast with previous studies on screening, participants were not selected for screening on the basis of the presence of symptoms; all health-care workers were offered to be tested. Limitations include the fact that most health-care workers had to fill in the questionnaire and report the result of the lateral flow assay by themselves.
The reduced sensitivity the point-of-care test might relate to low seroconversion or to the performance of the point-of-care test. Thus, testing of PCR-verified patients in our internal validation might be biased because these patients might not have developed a humoral response yet, which would result in an expected lower sensitivity and therefore we expect sensitivity related to the performance of the point-of-care test to be higher than the observed 82·5%. This sensitivity is similar to the manufacturer-reported 90·6% sensitivity. The severity of symptoms could have been a confounder because we had little data about the severity of symptoms among participants.
The prevalence of COVID-19 is low in Denmark, in accordance with the findings of this study. The point-of-care test will underestimate the seroprevalence but when adjusting for test characteristics including sensitivity, the result of the sensitivity analysis did not differ significantly. For individual patient assessment the sensitivity of a proper laboratory-based test is expected to be superior. We do, however, find the point-of-care test suitable for scientific use. By comparison, pharyngeal swabs have an even lower sensitivity.26 RRs also would not be affected because sensitivity is expected to be balanced between groups. In our study we found the specificity and validation sufficient. A confirmation assay (eg, plaque reduction neutralisation test) was not feasible in a cohort of this size.
Reporting of symptoms was high because many reported a single symptom such as sneezing. Because participants were not masked to test results when filling in the questionnaire, knowing the test result might have introduced bias of symptom reporting. Another potential bias could be that symptomatic and infected health-care workers stayed at home. We therefore allowed health-care workers who were not able to come to the hospital during the screening to complete the questionnaire at home. Our experience is that the desire to be tested was very high. Still, healthy health-care workers were possibly less likely to be tested than those with symptoms of infection, which might introduce a bias. Transmission can emerge from patient to health-care worker interaction but also between health-care workers. We do not have the information to answer this important question, which prompts further research.
We used information on seropositivity from all voluntary blood donations in the same region as a control group for this study. In this context blood donors represented the general population of a similar age with the limitations that must be considered. Blood donors are healthy individuals screened for behavioural risks such as travelling abroad or having a fever. Seropositivity might be lower in this group compared with the general population.
In conclusion, we found an overall low seroprevalence of SARS-CoV-2 antibodies in health-care workers. Seroprevalence was, however, higher than in blood donors. Frontline health-care workers working in hospitals and health-care workers working on a dedicated COVID-19 ward had significantly higher seroprevalence rates than other health-care workers did. More than half of seropositive health-care workers had symptoms attributable to SARS-CoV-2.
This online publication has been corrected. The corrected version first appeared at thelancet.com/infection on August 14, 2020
Acknowledgments
Acknowledgments
This study was funded by the Lundbeck Foundation (R349-2020-731). The Livzon tests were donated by Bestseller Foundation. We want to thank the directors of the Capital Region of Denmark (Region H) for their support of the study. Also, we want to express our gratitude to the hospital boards for their support and all the heads of clinical hospital departments in Region H who arranged for the testing of staff and without whom this study would not have been possible. We are also very grateful to all of the staff at the local clinical biochemistry departments who made the sample and testing logistics possible with only very limited time for preparation.
Contributors
The study was designed and initiated by the steering committee: KI, HB, OA, TKF, CAJJ, CT-P, RM, JR, FF, CS, TB, SDN, and HU. Data was collected by RBH, JHK, PBN, MP-H, ADK, CEC, KF, JBN, SBD, CEH, MG-B, ES, and LH. RBH, JHK, CEH, KI, HB, and HU analysed the data. The first draft was written by JHK, RH, KI, HB, and HU. All authors critically revised the manuscript and agree to be accountable for all aspects of the work.
Declaration of interests
CT-P reports grants from Bayer and Novo Nordisk, outside of the submitted work. CEC reports grants from Lundbeck Foundation and personal fees from Teva pharmaceuticals, outside of the submitted work. SDN reports grants from Novo Nordic Foundation, during the conduct of the study. TB reports grants from Pfizer, Novo Nordisk Foundation, Simonsen Foundation and Lundbeck Foundation; grants and personal fees from GSK, Pfizer, and Gilead; and personal fees from Boehringer Ingelheim, and MSD, outside of the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.WHO Coronavirus disease (COVID-19): situation report 179. July 17, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200717-covid-19-sitrep-179.pdf?sfvrsn=2f1599fa_2
- 2.Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Instituto de Salud Carlos III Informe sobre la situación de COVID-19 en España. https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/Informes COVID-19/Informe n° 20. Situación de COVID-19 en España a 3 de abril de 2020.pdf
- 4.International Council of Nurses High proportion of healthcare workers with COVID-19 in Italy is a stark warning to the world: protecting nurses and their colleagues must be the number one priority. March 20, 2020. https://www.icn.ch/news/high-proportion-healthcare-workers-covid-19-italy-stark-warning-world-protecting-nurses-and
- 5.Long Q-X, Liu B-Z, Deng H-J, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 6.Wajnberg A, Mansour M, Leven E, et al. Humoral immune response and prolonged PCR positivity in a cohort of 1343 SARS-CoV 2 patients in the New York City region 2. medRxix. 2020 doi: 10.1101/2020.04.30.20085613. published online May 5. (preprint) [DOI] [Google Scholar]
- 7.Kåre Mølbak: Social afstand virker så godt, at vi kan undvære flokimmunitet, Jyllands-Posten. April 15, 2020. https://jyllands-posten.dk/indland/ECE12077695/kaare-moelbak-social-afstand-virker-saa-godt-at-vi-kan-undvaere-flokimmunitet/
- 8.Kwok KO, Lai F, Wei WI, Wong SYS, Tang JWT. Herd immunity—estimating the level required to halt the COVID-19 epidemics in affected countries. J Infect. 2020;80:e32–e33. doi: 10.1016/j.jinf.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day M. Covid-19: four fifths of cases are asymptomatic, China figures indicate. BMJ. 2020;369 doi: 10.1136/bmj.m1375. [DOI] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogan WJ, Gladen B. Estimating prevalence from the results of a screening test. Am J Epidemiol. 1978;107:71–76. doi: 10.1093/oxfordjournals.aje.a112510. [DOI] [PubMed] [Google Scholar]
- 13.WHO COVID-19 Public Health Emergency of International Concern (PHEIC) global research and innovation forum. Feb 12, 2020. https://www.who.int/publications/m/item/covid-19-public-health-emergency-of-international-concern-(pheic)-global-research-and-innovation-forum
- 14.Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of COVID-19—studies needed. N Engl J Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 15.Sundhedsstyrelsen (Danish Health Authoritiy) The first Dane with COVID-19 is doing well and is in home isolation. Feb 27, 2020. https://www.sst.dk/da/Nyheder/2020/Foerste-dansker-med-COVID-19-har-det-godt_-og-er-i-hjemmeisolation/The-first-Dane-with-COVID-19-is-doing-well-and-are-in-home-insulation
- 16.Tostmann A, Bradley J, Bousema T, et al. Strong associations and moderate predictive value of early symptoms for SARS-CoV-2 test positivity among healthcare workers, the Netherlands, March 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.16.2000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keeley AJ, Evans C, Colton H, et al. Roll-out of SARS-CoV-2 testing for healthcare workers at a large NHS Foundation Trust in the United Kingdom, March 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.14.2000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter E, Price DA, Murphy E, et al. First experience of COVID-19 screening of health-care workers in England. Lancet. 2020 doi: 10.1016/S0140-6736(20)30970-3. published online April 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivett L, Sridhar S, Sparkes D, et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020;9 doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutton D, Fuchs K, D'Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;82:2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roxby AC, Greninger AL, Hatfield KM, et al. Detection of SARS-CoV-2 among residents and staff members of an independent and assisted living community for older adults—Seattle, Washington, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:416–418. doi: 10.15585/mmwr.mm6914e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baggett TP, Keyes H, Sporn N, Gaeta JM. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020 doi: 10.1001/jama.2020.6887. published online April 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.COVID-19-smittet var til fredagsbar på Panum Instituttet i København fredag 6. marts. March 10, 2020. Styrelsen for Patientsikkerhed. https://stps.dk/da/nyheder/2020/covid-19-smittet-var-til-fredagsbar-paa-panum-instituttet-i-koebenhavn-fredag-6-marts/?fbclid=IwAR3LEpcxTbFKqQeongR0MTq5c9V7ElLK_Vyw30fuWJc4e77YIS4FlWspaTY#
- 24.European Centre for Disease Prevention and Control; April 8, 2020. Coronavirus disease 2019 (COVID-19) in the EU/EEA and the UK—eighth update.https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-rapid-risk-assessment-coronavirus-disease-2019-eighth-update-8-april-2020.pdf [Google Scholar]
- 25.Statens Serum Institut COVID-19 i Danmark: epidemiologisk overvågningsrapport. April 25, 2020. https://files.ssi.dk/COVID19-overvaagningsrapport-25042020-sr21
- 26.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.