Figure 2. Leptin increases SENP2 expression through the leptin receptor/STAT3 pathway.
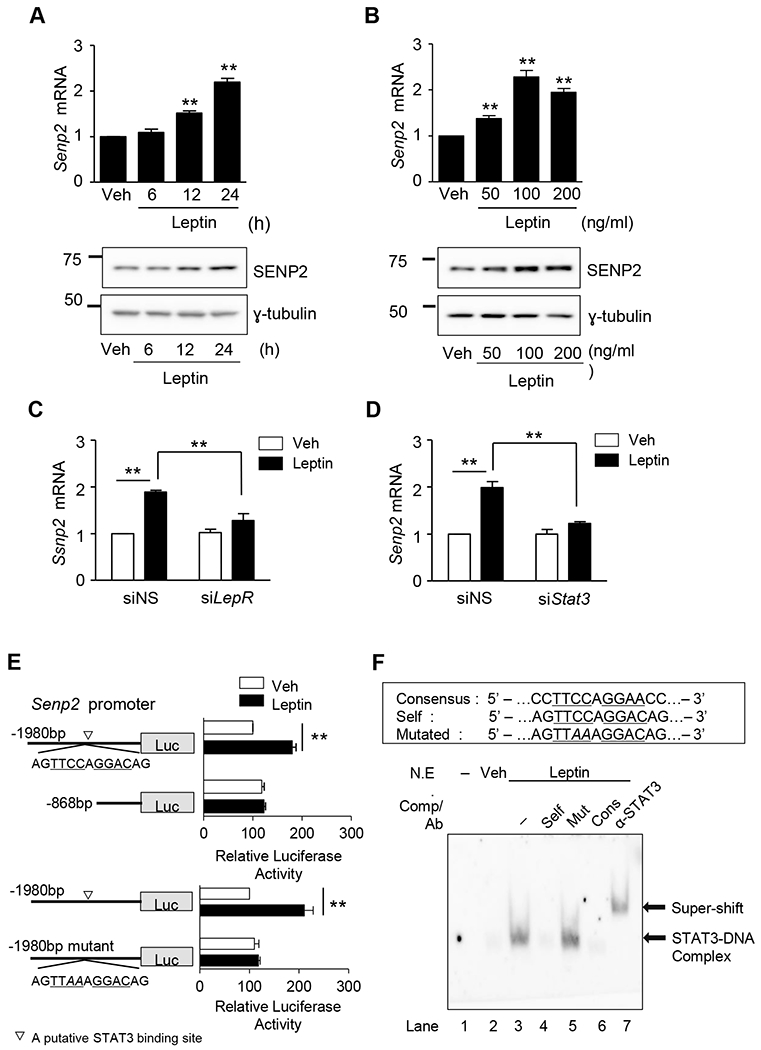
(A) C2C12 myotubes were treated with leptin (100 ng/mL) for different periods. The mRNA level of Senp2 was measured by real time PCR (upper panel). The mRNA level of leptin-untreated cells (Veh) was expressed as 1, and the others were expressed as its relative values (n=4). Western blotting was also performed using an anti-SENP2 antibody (lower panel). (B) C2C12 myotubes were treated with various concentration of leptin for 24 h, and real time PCR (upper panel) (n = 4) and western blotting (lower panel) were performed. (C, D) C2C12 myotubes were treated with siRNAs (100 nmol/1) against leptin receptor (siLepR) (C) or STAT3 (siStat3) (D) for 24 h and then Senp2 mRNA levels were measured (n = 4). (E) C2C12 myoblasts were transfected with one of the Senp2 promoter-Luc constructs and then treated with leptin (100 ng/ml) for 24 h. Normalized luciferase activity of the cells, transfected with mSenp2(−1980)-Luc without leptin, was expressed as 100 and the others were expressed as its relative values (n = 5). A putative STAT3 binding site was indicated (▽). *P < 0.05, **P < 0.01. Data are presented as mean ± SEM. (F) Oligonucleotides (Self) representing the STAT3 binding site identified in Fig. 2E were labeled with biotin and incubated with nuclear extracts of C2C12 myotubes treated with leptin. Unlabeled oligonucleotides (a 50-fold excess) (Self, mutated [Mut], consensus [Cons]) were used as competitors (Comp), and an anti-STAT3 antibody was used for super-shift. Lane 1, the probe alone.
