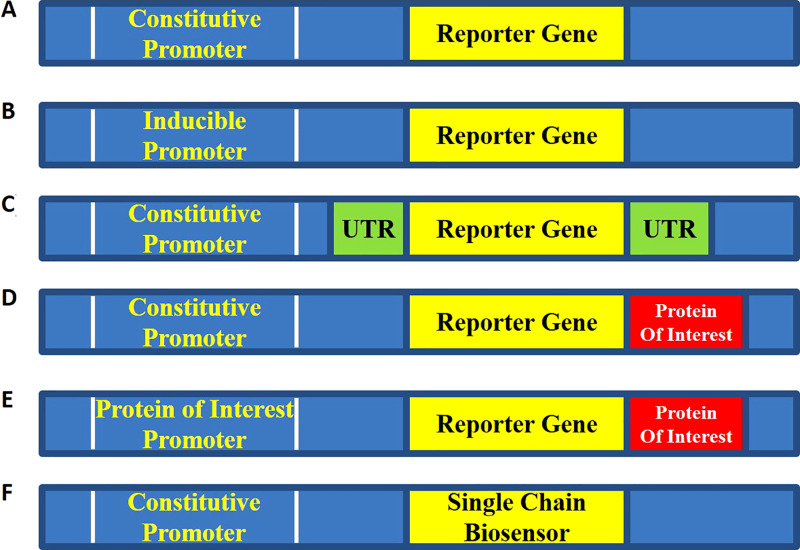
Figure 2:
Typical configurations of genetically encoded imaging reporters. A, Constitutive reporter. The reporter is under the control of a constitutive promoter such as simian virus 40, chicken β-actin, or cytomegalovirus promoter. This design is most often utilized for cell tracking or trafficking experiments. B, Regulated transcriptional reporter. This design is utilized to monitor promoter activity in various cell types under various stimuli. Control constructs such as (A) or constructs with empty promoter regions are required to demonstrate specificity of signal induction. C, Translational or posttranscriptional reporters. The untranslated region (UTR) of interest is included either upstream or downstream of the reporter under the control of a constitutive promoter. Constructs such as (A) or mutated UTR are required to demonstrate specificity of signal change. D, Posttranscriptional reporters. The reporter gene is fused to a protein of interest typically through a glycine-serine linker region. As the protein of interest is degraded or trafficked, the reporter is concomitantly degraded or trafficked through the cell. Key controls involve fusing to a mutated form of the protein of interest that is no longer degraded or appropriately trafficked through the cell. E, Feedback-regulated reporter. In this case, the fusion reporter from (D) is also controlled by the promoter of the gene of interest. The feedback-regulated dynamics of degradation and synthesis can then be studied. F, Biosensor. A modified luciferase or fluorophore that changes either brightness or spectral output in response to changes in the local environment is developed. This construct is placed under the control of a constitutive promoter. Constructs such as (A) or modified biosensors with point mutations no longer capable of sensing environmental changes are required to demonstrate specificity.