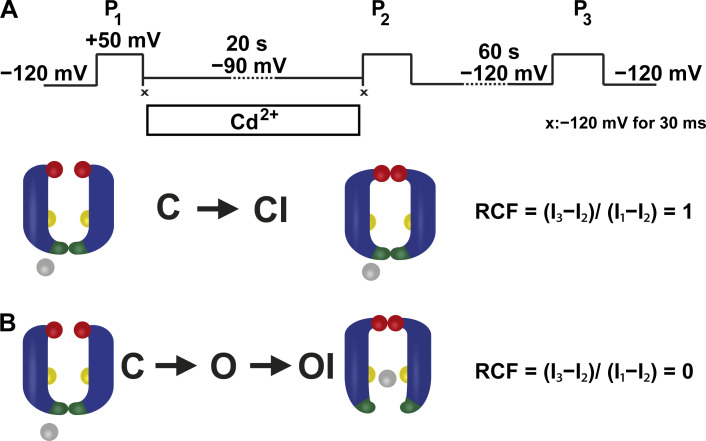
Figure 3.
Experimental strategy to investigate closed-state inactivation in T449A/V474C Shaker-IR ion channels. (A) The pulse protocol consisted of three 5-ms-long depolarizations to +50 mV (P1, P2, and P3). The corresponding peak currents evoked by these pulses are represented as I1, I2, and I3 in B. Between P1 and P2, patches were held in Cd2+ containing intracellular solution at −90 mV holding potential for 20 s. To avoid the Cd2+ exposure of the open-state channels during P1 and P2, 30-ms-long steps to −120 mV were applied directly after P1 and before P2, indicated by symbol ×. The holding potential of −120 mV was applied between P2 and P3 for 60 s. (B) The accessibility of cysteines at position 474 (yellow spheres) located between the activation (intracellular part of S6 helices, dark green) and C-type inactivation (red spheres) gates during inactivation was determined by applying Cd2+ (gray spheres) from the intracellular side. Upon binding to 474C, Cd2+ inhibits K+ currents, thus tracking precisely the incidental opening of the A-gate during the development of C-type inactivation. Model predictions for RCF are in the text; I1, I2, and I3 are defined in A.