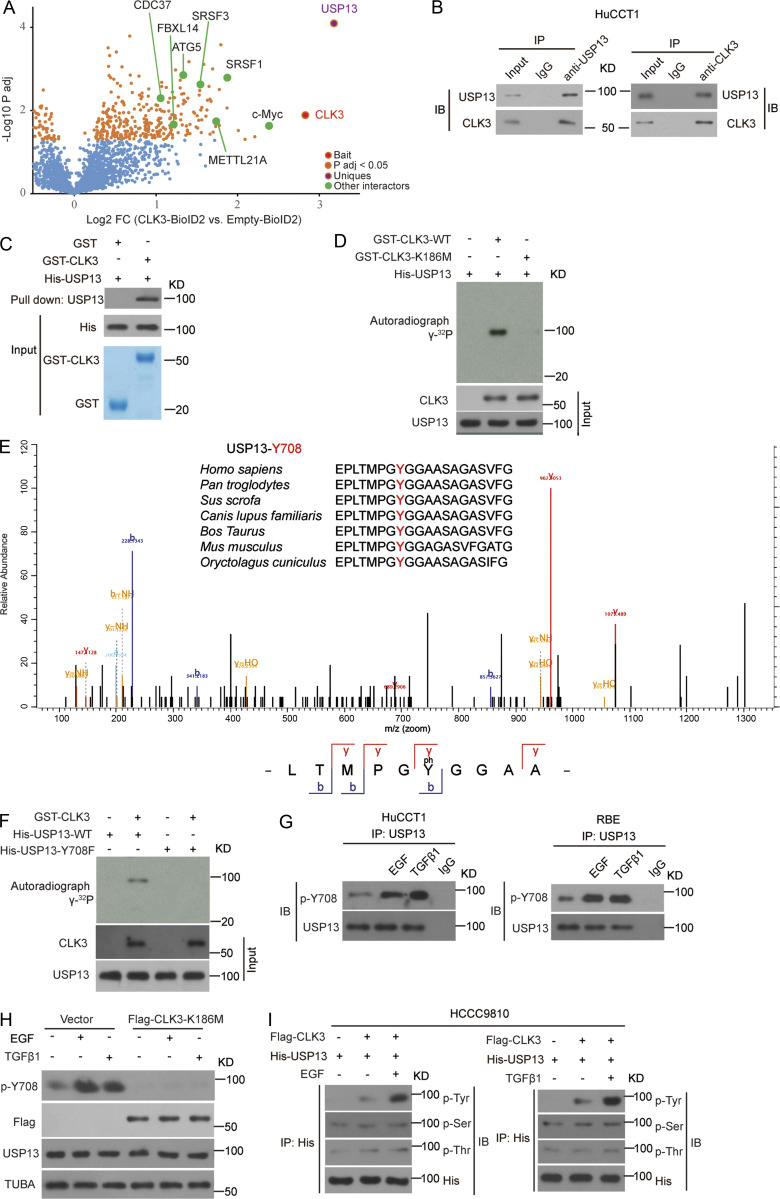
Figure 5.
CLK3 directly interacts with and phosphorylates USP13 at Y708. (A) Volcano plot showing CLK3 interactors identified in HCCC9810 cells with Empty-BioID2 or CLK3-BioID2 (n = 6). (B) Co-IP assays for endogenous CLK3 and USP13 from HuCCT1 cells. IB, immunoblot. (C) GST pulldown assays were performed. (D) Ni-NTA agarose beads were used to immobilize bacterially purified His-CLK3 proteins as indicated. Then, these beads were incubated with purified GST-USP13 and [γ-32P] ATP kinase buffer. Autoradiography was performed. (E) MS was performed to identify CLK3-induced phosphorylation site of USP13 (lower panel). Sequences containing Y708 across species are shown (upper panel). (F) The effect of USP13-Y708F on CLK3-mediated phosphorylation. (G) HuCCT1 and RBE cells were treated with or without EGF or TGFβ1. IP with USP13 was performed. A specific anti-phospho-USP13-Y708 antibody produced by this group was used to detect USP13 phosphorylation. (H) HuCCT1 cells with or without CLK3-K186M transfection were treated by EGF (50 ng/ml) or TGFβ1 (10 ng/ml). IP with USP13 was performed. A specific anti-phospho-USP13-Y708 antibody was used to detect USP13 phosphorylation. (I) HCCC9810 cells stably expressing Flag-CLK3 and His-USP13 were treated with EGF (50 ng/ml) or TGFβ1 (10 ng/ml) for 60 min. Western blots were performed as indicated. Phosphotyrosine (p-Tyr), phosphoserine (p-Ser), and phosphothreonine (p-Thr) were analyzed. Data are representative of three independent experiments with similar results (B–D and F–I). TUBA, alpha tubulin.