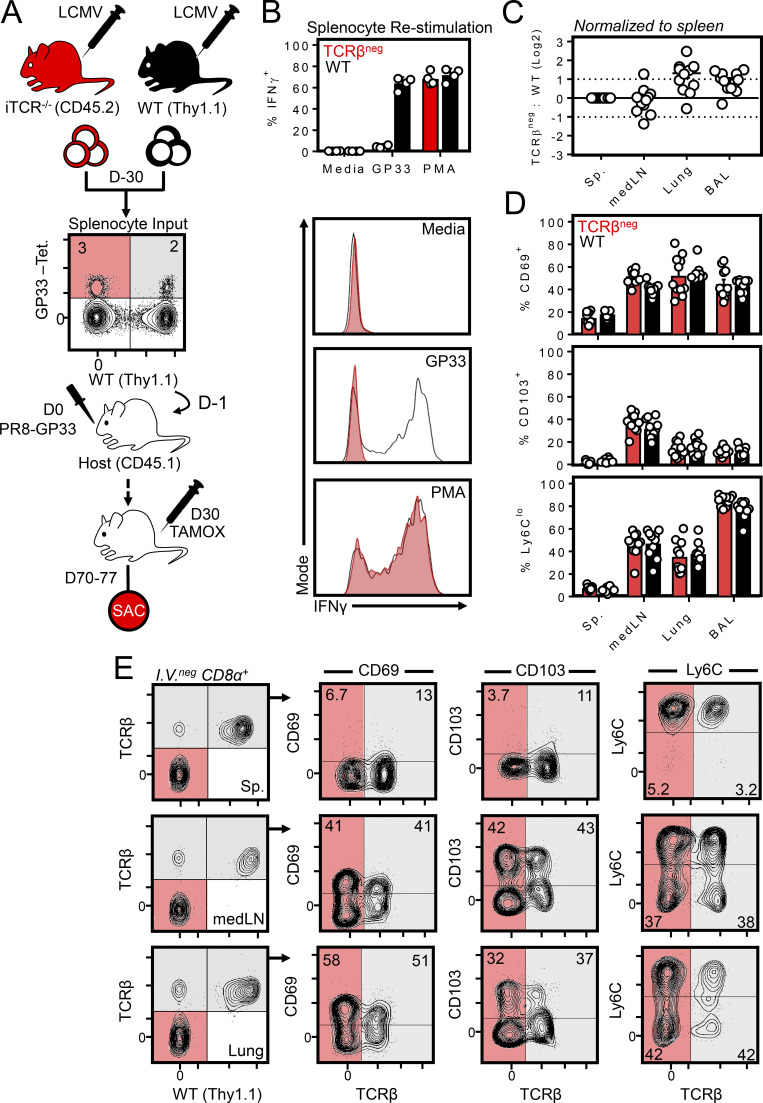
Figure 4.
MedLN TRM are maintained independently of TCR. (A) Experimental strategy for generating gp33-specific WT and TCRβneg cells from a polyclonal repertoire. WT and ub-CreERT2 × TracFL mice (referred to here as iTCR−/− mice) were infected with LCMV i.p., and splenocytes were isolated 30 d later and combined such that the frequency of tetramer+ gp33-specific CD8+ T cells of WT or iTCR−/− origin were ∼1:1. Red shaded quadrants indicate iTCR−/−-derived tetramer+ cells; gray shaded quadrants represent WT-derived tetramer+ cells. Cells were cotransferred into congenically distinct recipient mice subsequently infected with PR8-gp33 i.n. to establish secondary memory medLN TRM from both donor sources. 30 d later, mice were treated with tamoxifen daily for 7 d to ablate the TCR specifically from iTCR−/−-derived cells. Mice were sacrificed (SAC) 40 d after the induction of tamoxifen treatment. (B) In vitro peptide stimulation of WT and iTCR−/− cocultures confirmed TCRβneg cells were refractory to peptide reactivation but could produce IFNγ through a TCR-independent mechanism (PMA). Data are from one of two independent experiments with similar results. (C) Ratio of TCRβneg: WT cells in the spleen, medLN, lung, and brochoalveolar lavage. (D and E) Frequency of CD103+, CD69+, and Ly6Clo cells among transferred WT or TCRβneg memory CD8+ T cells within the indicated tissues. Gray shade indicates WT cells; red indicates iTCR−/− cells in which the TCR was successfully ablated (as indicated by an absence of TCRβ). iTCR−/−-derived cells that did not undergo loss of TCR expression after tamoxifen treatment were included within analysis of WT cells. C and D pooled from 10–12 mice from three independent experiments; E shows representative flow cytometry plots. Bars represent mean ± SEM.