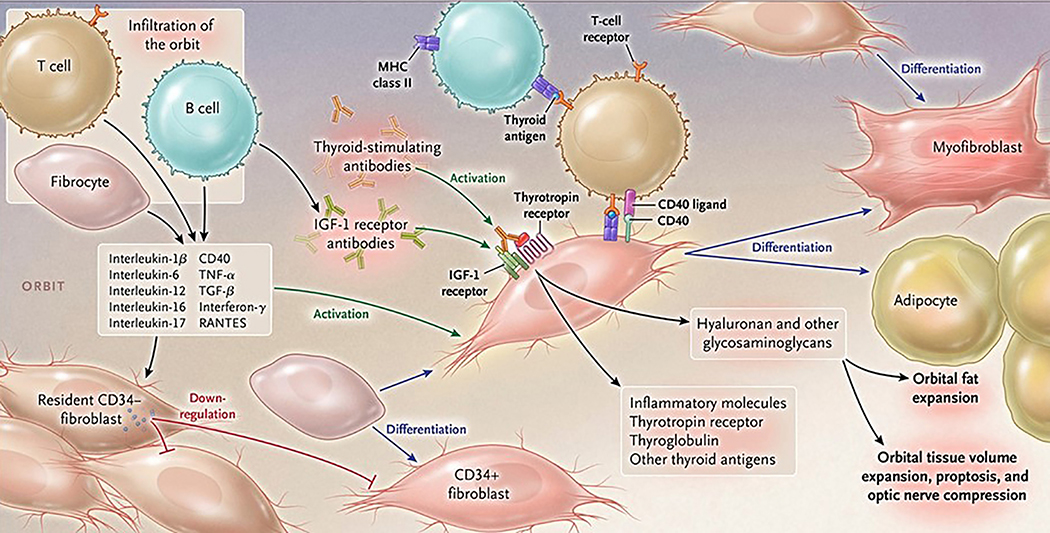
Figure 2. Proposed theoretical model of TAO pathogenesis.
Bone marrow-derived CD34+ fibrocytes circulatate in Graves’ disease at higher levels. They express several thyroid autoantigens, including thyrotropin receptor (TSHR), thyroglobulin, thyroperoxidase and sodium-iodide symporter. Fibrocytes present antigens to T cells through Class 2 MHC. They endorse B cell production of IgG1, can differentiate into CD34+ fibroblasts, myofibroblasts and adipocytes. CD34+ fibroblasts are held in check by residential CD34- fibroblasts. When activated, CD34+ fibroblasts generate several pro-inflammatory or anti-inflammatory cytokines, including interleukins 1β, 6, 8, 10, 12, 16, tumor necrosis factor α, and regulated on activation, normal T expressed and secreted (RANTES), CXCL-12 and CD40 CD154. Both infiltrating and residential cells express insulin-like growth factor-I receptor (IGF-IR), suggesting that this tyrosine kinase might represent a therapeutic target. Alternatively, levels and actions of cytokines could be attenuated. Fibroblasts synthesize hyaluronan, potentially expanding tissue volume. Orbital fat can also expand in TAO. From N. Engl. J. Med, Smith T.J. and Hegedus L., Graves’ Disease, 375; 1552–1565. Copyright © (2016) Massachusetts Medical Society. Reprinted with permission.