Figure 1. Overview of mechanisms linked to AF-occurrence.
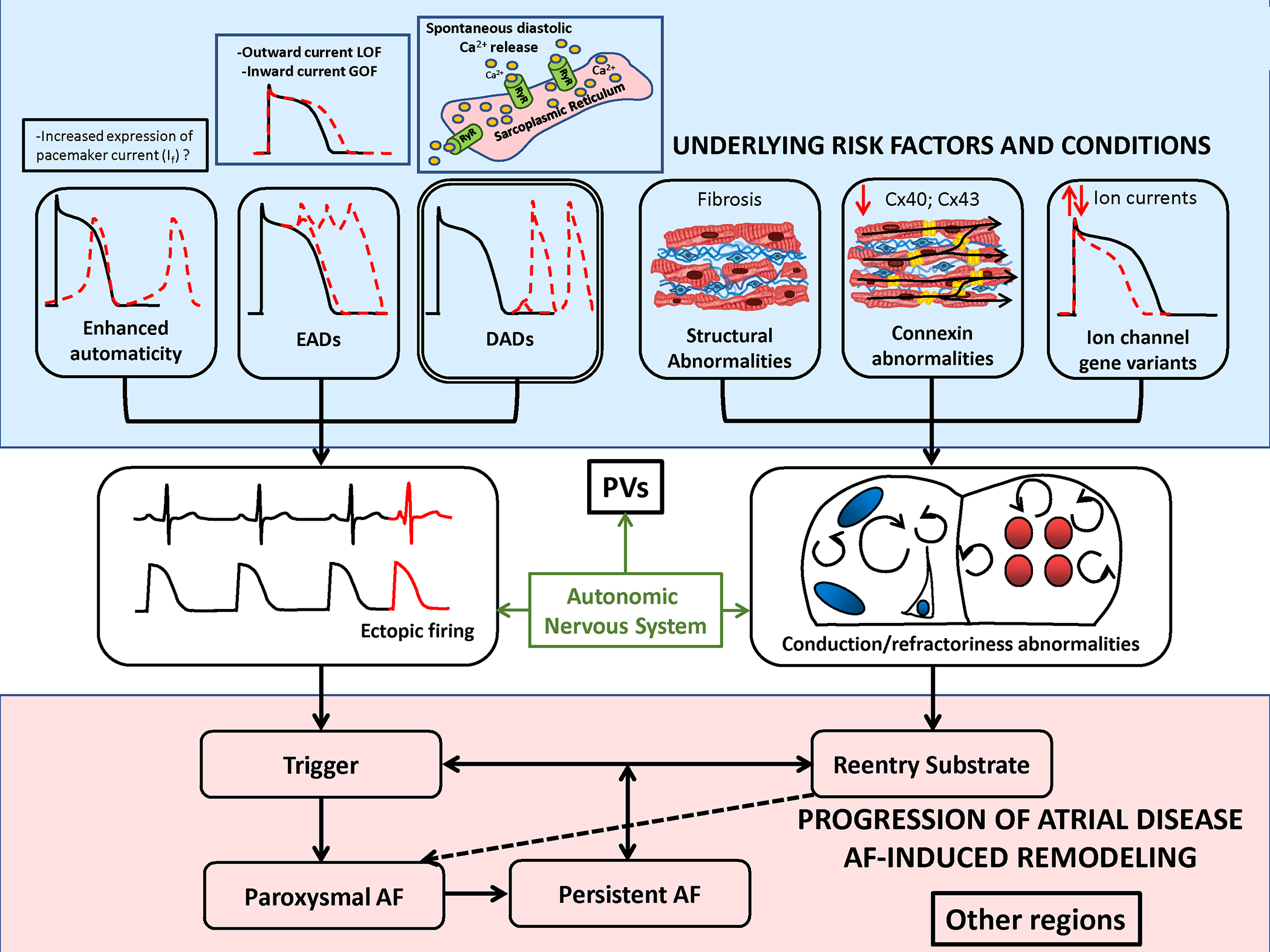
AF AF-triggers result from focal ectopic firing. Ectopic activity is most clearly linked to spontaneous diastolic Ca2+-release from the sarcoplasmic reticulum Ca2+-stores via leaky ryanodine-receptor (RyR2) Ca2+-release channels. Early afterdepolarizations (EADs) due to loss-of-function (LOF) outward-current mutations or gain-of-function (GOF) inward-current mutations have also been linked to spontaneous ectopy. Enhanced automaticity, for example due to pacemaker current expression, is another possible cause of ectopic activitty, but has not been definitively demonstrated. AF-persistence is linked to AF-maintaining reeentry that requires both trigger and a vulnerable reentrant substrate. The latter can be caused by abbreviated refractoriness (e.g. due to a GOF K+-channel mutation or to enhanced vagal tone) or by conduction abnormalities due to tissue fibrosis, connexin (Cx) dysfunction or LOF Na+-channel mutations. Ectopic firing typically originates from the pulmonary veins (PVs), but the PVs are also a priviledged site for reentry susceptibility.
