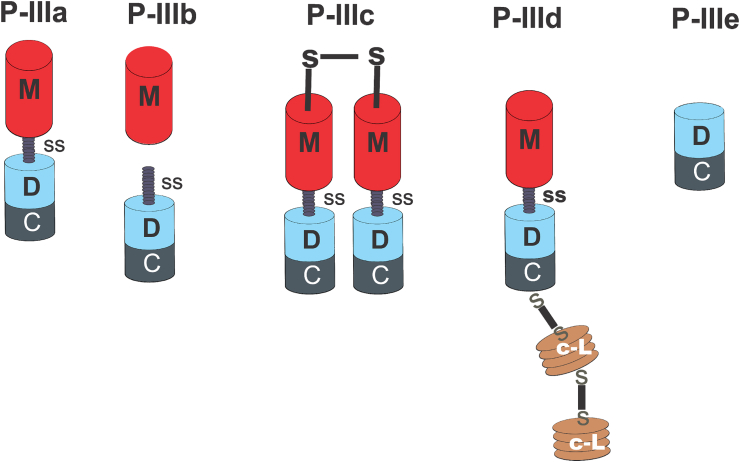
Fig. 3.
Classification of the P-III SVMPs. P-IIIa has a metalloproteinase (M), a disintegrin-like (D) and a cysteine-rich (C) domain, displaying the canonical structure of P-III SVMPs. The D adjacent to the M domain of P-IIIb subclass is vulnerable to proteolytic cleavage. Members of the P-IIIc subclass form homo- or heterodimers connected by a disulfide bridge formed between the M domains of the two monomers. The P-IIId subclass represents a complexed P-III SVMP in which the C-terminal of the cysteine-rich domain is covalently linked to a tandem arrangement of two covalently bridged Snake C-type lectin-like regions-Snaclecs (c-L). The M domain is absent in the novel P-IIIe subclass and the processed form is a didomain containing D and C domains.