Figure 5:
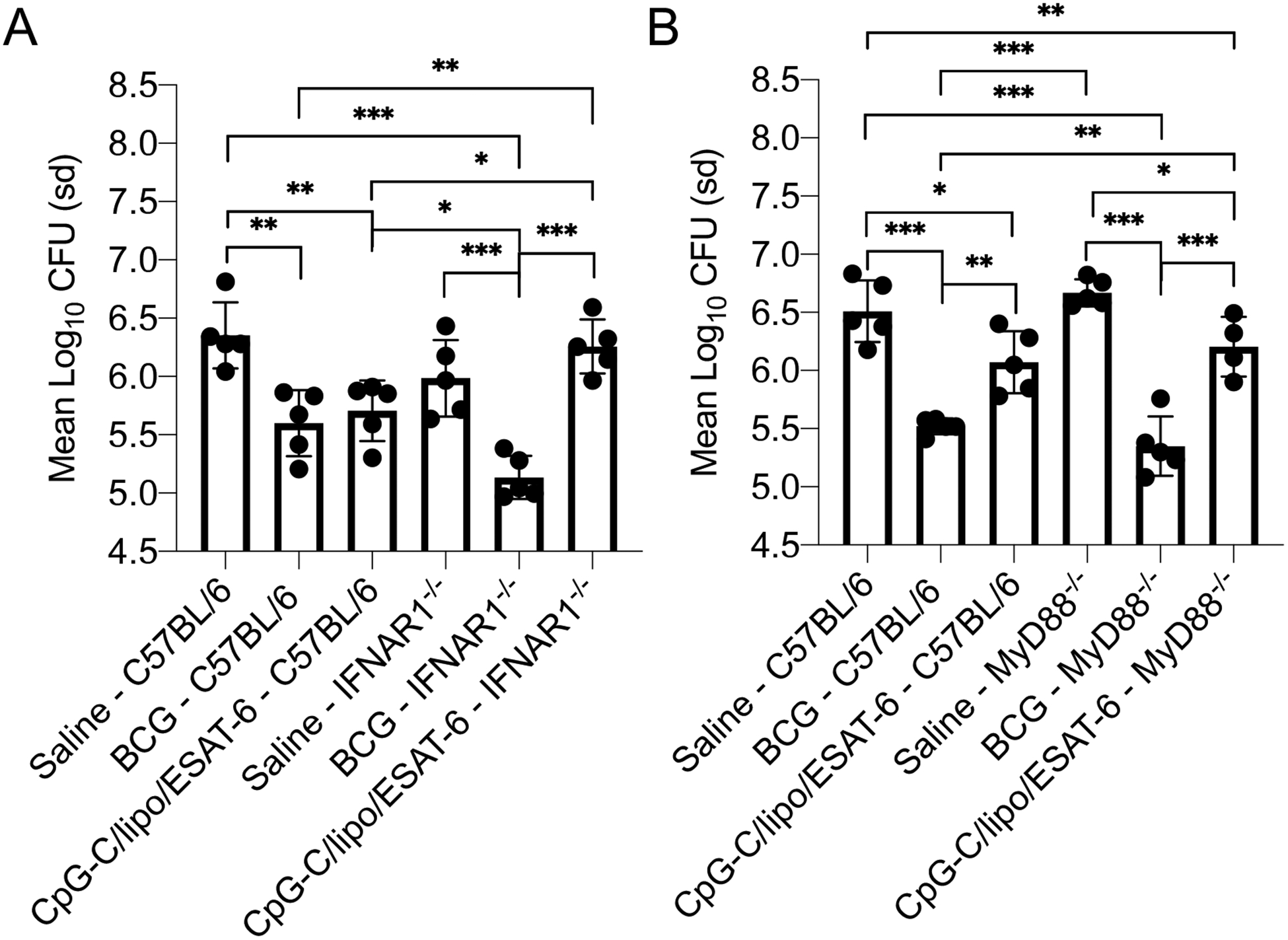
(A) The mycobacterial burden in the lungs of IFNAR1−/− mice and C57BL/6 mice inoculated with either BCG via the subcutaneous route or CpG-C/liposome/ESAT-6 formulation via the intranasal route at day 30 after the final inoculation. Mice were vaccinated with BCG via the subcutaneous route. The mycobacterial burden was determined at day 30 post pulmonary infection with M. tuberculosis H37Rv. (B) The mycobacterial burden in the lungs of MyD88−/− mice and C57BL/6 mice inoculated with either BCG via the subcutaneous route or CpG-C/liposome/ESAT-6 via the intranasal route at day 30 after the final inoculation. Data were analyzed using the One-way ANOVA for independent measures with post-hoc Tukey test. Data are representative of two experiments. N = 4–5 mice per group. * = p<0.05, ** = p<0.01, *** = p<0.001
