FIG 6.
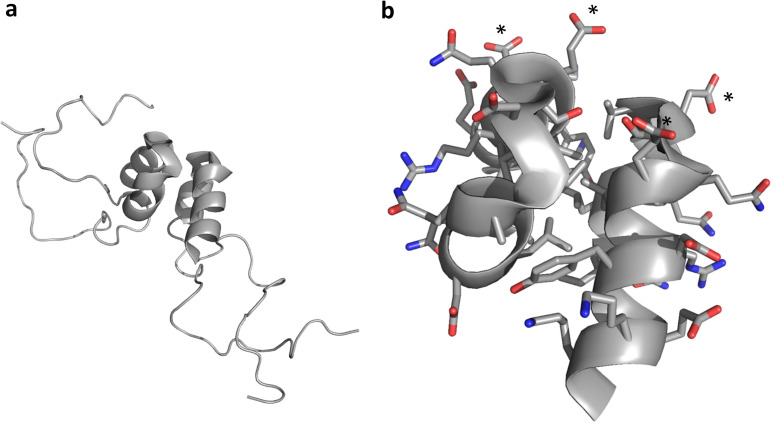
3A-N structure. (a) A soluble N-terminal 59-residue fragment of the PV1 3A protein forms a symmetric homodimer in solution. Each monomer contains a helical hairpin, flanked by largely disordered N and C termini. (b) Zoomed view of the 3A-N dimer interface, showing a hydrophobic dimerization surface that includes residues I-22, L-25, L-26, V-29, V-34, Y-37, C-38, and W-43. The nondimer surfaces are highly charged, with a negative charge cluster (D-29 and E-32 from each monomer) indicated by asterisks. Side chain oxygen atoms are shown in red. Side chain nitrogen atoms are shown in blue.