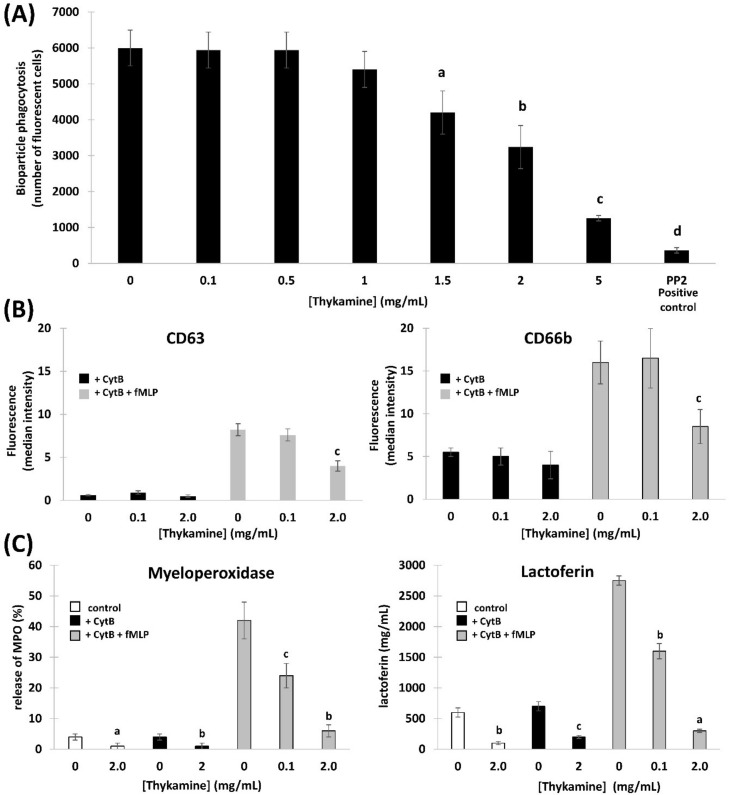
Figure 4.
In vivo effect of Thykamine on phagocytosis (A) and on granule functions (B,C) in normal human blood neutrophils. (A) Phagocytosis of fluorescein-labeled Escherichia coli BioParticles by normal human blood neutrophils. Neutrophils pretreated 30 min with graded concentrations of Thykamine (or HBSS as negative control, and 10 µM phosphoprotein phosphatase PP2 as positive control) were incubated 30 min at 37 °C in the presence of homogenized FITC-labeled E. coli BioParticles. Trypan blue was added to quench the extracellular fluorescence of non-phagocytosed E. coli BioParticles before analysis by cytofluorometry (485 nm excitation, 530 nm emission wavelengths). Results are expressed in number of fluorescent neutrophils that have phagocytized fluorescent particles among 10,000 cells analyzed. Statistics were performed using Student’s unpaired t test (comparison between Thykamine-treated neutrophils versus control neutrophils in HBSS) of n = 7 where significance for p value is a: p < 0.001; b: p < 0.005; c: p < 0.0001 and d: p < 0.0005. (B,C) Release of primary and secondary granules by normal human blood neutrophils.Neutrophils were pretreated 30 min with 0.1 or 2 mg/mL Thykamine (or HBSS as negative control) before stimulation with vehicle or 10−7 M fMLP for 5 min at 37 °C in the presence or absence of 1 µg/mL cytochalasin B (CytB) with or without fMLP. Statistics were performed using Student’s unpaired t test (comparison between Thykamine-treated versus control cells in HBSS) where significance for p value is a: p < 0.001; b: p < 0.005 and c: p < 0.01. (B) Degranulation was monitored by the appearance of CD63 (primary granules) and CD66b (secondary granules) at the cell surface, as analyzed by cytofluorometry. Results are expressed in arbitrary units of fluorescence intensity (mean ± SEM; n = 3). (C) Degranulation was assessed by the release in supernatants of MPO and LF. MPO was evaluated by comparison to a calibration curve created with known dilutions of human MPO. LF was measured by ELISA using a peroxidase-conjugated anti-rabbit Ab. Results of MPO and LF are expressed in percentages (ratio extra-cellular/intra- + extra-cellular materials) and in ng/mL, respectively.